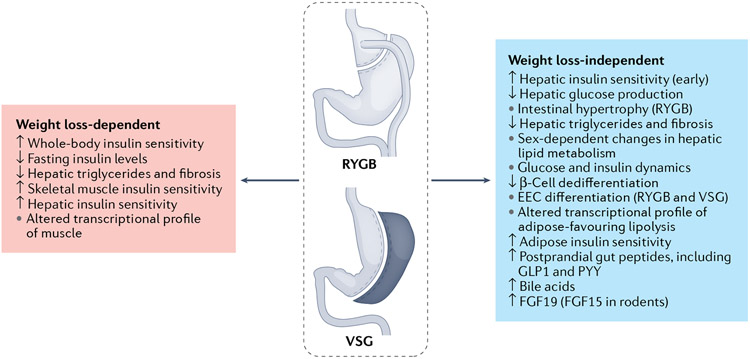
Fig. 1 ∣. Weight loss-dependent and weight loss-independent mechanisms of bariatric surgery on glucose metabolism.
Bariatric surgery has important weight loss-dependent and weight loss-independent mechanisms for driving improvements in glucose homeostasis. In itself, weight loss improves whole-body and specifically muscle and adipose insulin sensitivity. By contrast, the liver seems to be more susceptible to weight loss-independent effects on improving hepatic insulin sensitivity. Weight loss-independent effects also include changes in postprandial glucose and insulin dynamics, with high peaks and rapid returns towards baseline. Bariatric surgery leads to increases in postprandial levels of multiple gut peptides, bile acids and fibroblast growth factor 19 (FGF19; FGF15 in rodents). Changes in the nutrient profile and increases in bile acid signalling contribute to increases in intestinal cell proliferation (Roux-en-Y gastric bypass (RYGB)) and differentiation towards the enteroendocrine cell (EEC) lineages (both RYGB and vertical sleeve gastrectomy (VSG)). There are probably combined weight loss-dependent and weight loss-independent effects on hepatic levels of triglycerides. GLP1, glucagon-like peptide 1; PYY, peptide YY.