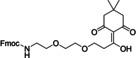
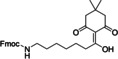
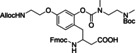
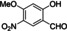
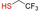Table 3.
Synthetic methods used in chemical protein synthesis, indicating use in reported D‐protein synthesis and potential issues encountered with use.
|
Entry |
|
|
|
Used in D‐protein synthesis |
|
|---|---|---|---|---|---|
|
# |
Synthetic method |
Reagents |
Y / N |
Potential issues |
Ref. |
|
|
Ligation method |
|
|
|
|
|
|
|
|
|
|
|
|
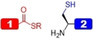
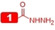
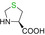
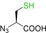
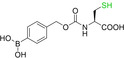
1 |
Native chemical ligation |
+ thiol catalyst |
Y |
Dependence on suitable cysteine or alanine residues. |
[97a] |
|
|
|
|
|
|
|
|
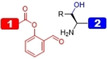
2 |
Serine/threonine ligation |
+ acidolysis |
N |
Requires suitable Ser/Thr. Slower reaction kinetics than NCL. |
[145] |
|
3 |
KAHA ligation |
|
N |
Accessibility of enantiomeric reagent. |
[146] |
|
|
|
|
|
|
|
|
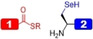
4 |
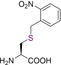
Selenocysteine NCL |
+ thiol catalyst |
N |
Accessibility of enantiomeric reagent. |
[123a] |
|
|
NCL reactive end |
|
|
|
|
|
|
Thioester surrogate |
|
|
|
|
|
|
|
|
|
|
|
|
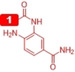
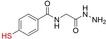
5 |
Hydrazides |
Activation+NaNO2 + Thiol |
Y |
Oxidation incompatible with Thz. Low temperature activation needed (<−15 °C). |
[100] |
|
|
|
|
|
|
|
|
6 |
Dbz |
Activation 4‐nitrophenyl chloroformate or NaNO2 or isoamyl nitrite +thiol |
Y |
Di‐acylation side product with excess Gly. |
[16, 147] |
|
|
|
|
|
|
|
|
7 |
MeDbz |
Activation 4‐nitrophenyl chloroformate +thiol |
N |
Difficult to activate off‐resin. |
[98] |
|
|
|
|
|
|
|
|
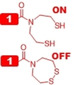
8 |
SEA |
Activation of SEA OFF TCEP+thiol |
N |
Latent SEAOFF thioester incompatible with TCEP during ligations. |
[148] |
|
|
N‐cysteine protection |
|
|
|
|
|
|
|
|
|
|
|
|
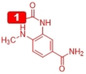
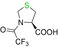
9 |
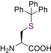
Thz |
Deprotection MeONH2 |
Y |
Incompatible with hydrazide oxidation. |
[103] |
|
|
|
|
|
|
|
|
10 |
Cys(Tfacm) |
Deprotection pH 11.5 |
N |
Accessibility of enantiomeric reagent. |
[149] |
|
|
|
|
|
|
|
|
11 |
TFA‐Thz |
Deprotection Base then MeONH2 |
Y |
Accessibility of enantiomeric reagent. |
[41] |
|
|
|
|
|
|
|
|
12 |
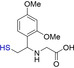
N3‐Cys |
Deprotection TCEP |
N |
Accessibility of enantiomeric reagent. |
[150] |
|
|
|
|
|
|
|
|
13 |
Cys(Dobz) |
Deprotection H2O2 |
N |
Accessibility of enantiomeric reagent. Harsh deprotection conditions. |
[151] |
|
|
Desulfurization |
|
|
|
|
|
14 |
Metal‐based |
Pd/Al2O3 Or Raney Nickel+ H2 (g) |
Y |
Removal of metal impurities can be problematic. Use of hydrogen gas. Potential side reactions with Trp and Met Quenched by thiol catalyst. Native Cys must be protected. |
[108] |
|
15 |
Metal‐free radical based |
VA‐044 TCEP tert‐butylthiol |
Y |
Quenched by thiol catalyst. Native Cys must be protected. |
[106] |
|
16 |
Beta/gamma thiol amino acids |
β‐thiol‐Phe β‐thiol‐Val β‐thiol‐Leu β‐thiol‐Asp β‐thiol‐Asn β‐thiol‐Arg γ‐thiol‐Val γ‐thiol‐Thr γ‐thiol‐Ile γ‐thiol‐Pro γ‐thiol‐Glu γ‐thiol‐Gln γ‐thiol‐Lys 2‐thiol‐Trp |
N |
Accessibility of enantiomeric reagent. Commercially available D‐Penicillamine (β‐thiol‐Val) could be used for D‐peptide ligation at Val, if directly following a glycine residue. |
[152] |
|
|
Thiol catalysts for one‐pot ligation‐desulfurization |
|
|
|
|
|
|
|
|
|
|
|
|
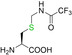
17 |
MPAA‐hydrazide |
pKa=6.6 Removal Aldehyde‐resin capture |
N |
Preparation of MPAA‐hydrazide reagent coupled with use in large excess is uneconomical. |
[153] |
|
|
|
|
|
|
|
|
18 |
Trifluoroethanthiol |
pKa=7.3 Removal Evaporation (bp=37 °C) |
N |
Malodorous and volatile, though could be used for D‐protein synthesis. |
[154] |
|
|
|
|
|
|
|
|
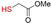
19 |
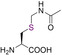
Methyl thioglycolate |
pKa=7.9 Removal none |
Y |
Slower kinetics with C‐terminal beta‐branched residue. |
[41] |
|
|
Solubility enhancers |
|
|
|
|
|
|
|
|
|
|
|
|
20 |
Helping hand v1 |
Installation Amine labelling with lysine side chain Removal Hydrazine (aq) |
N |
Additional steps to incorporate and remove tag. Potential issues with stability. |
[119a] |
|
|
|
|
|
|
|
|
21 |
Helping hand v2 |
Installation Amine labelling with lysine side chain Removal Hydrazine (aq) Or Hydroxylamine (aq) |
N |
Additional steps to incorporate and remove tag. |
[119b] |
|
|
|
|
|
|
|
|
22 |
Removable backbone modification v1 |
Installation Standard Fmoc‐SPPS Removal pH 7 then TFA |
Y |
Limited to Gly only. Lengthy synthesis of building block. Additional steps to incorporate and remove tag. |
[117, 155] |
|
|
|
|
|
|
|
|
23 |
Removable backbone modification v2 |
Installation Reductive amination Acetylation Removal Deacetylation (Cys (aq)) then TFA |
Y |
Additional steps to incorporate and remove tag. |
[77b, 118] |
|
|
Protein folding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disulfide #1 Removal TFA |
|
|
|
|
|
|
|
|
|
|
|
|
|
Disulfide #2 Removal Iodine or PdCl2 |
|
|
|
|
|
|
|
|
|
|
|
24 |
Cysteine orthogonal protection |
Disulfide #3 Removal UV light (350 nm) |
Y |
Practically limited to two disulfide bonds. Accessibility of a third, orthogonally protected D‐Cys building block. |
[52, 128] |
|
25 |
“Ambidextrous” chaperone |
GroEL/ES protein chaperone |
Y |
Mostly unnecessary for in‐vitro protein folding. Limited scope reported. |
[121b] |
|
|
Post‐translational modifications |
|
|
|
|
|
26 |
Lys ubiquitination |
δ‐mercapto lysine for NCL followed by desulfurization |
N |
Accessibility of enantiomeric reagent. |
[136a] |
|
27 |
Solid‐phase isopeptide bond formation |
N |
Requires assembly of large fragments by SPPS – not cost‐effective with D‐amino acids |
[156] |
|
|
|
|
|
|
|
|
|
28 |
Installation Coupling to lysine side chain PTM NCL to Ub‐thioester Auxiliary removal TFA |
N |
Low efficiency of ligation. Glycyl auxiliary replaced with Cys in preparation of enantiomeric di‐ and tri‐ubiquitin proteins. |
[28, 42] |
|
|
29 |
Lys trimethylation |
Fmoc‐Lys(Me3)‐OH |
N |
Accessibility of enantiomeric reagent. |
[133a, 151] |
|
30 |
Lys acetylation |
Fmoc‐Lys(Ac)‐OH |
N |
Accessibility of enantiomeric reagent. |
[133b] |
|
31 |
Asn N‐Glycosylation |
Fmoc‐Asn(Glycan)−OH or Boc‐Asn(Xan)−OH (and) further glycosylation on‐resin or in solution. |
N |
Accessibility of enantiomeric reagent (would also require L‐sugars). |
[53, 157] |
|
32 |
Oligosaccharide coupled directly to free Asn side chain during Boc‐SPPS. |
N |
Accessibility of enantiomeric reagent (would also require L‐sugars). |
[158] |
|
|
33 |
Thr O‐Glycosylation |
Fmoc‐Thr(Glycan)−OH |
N |
Accessibility of enantiomeric reagent (would also require L‐sugars). |
[131b] |
|
34 |
Cys S‐palmitoylation |
Fmoc‐Cys(Mmt)−OH Mmt removal on‐resin with 2 % TFA Reaction with palmitic anhydride |
N |
Incompatible with NCL. Potentially viable for D‐protein synthesis via STL or Sec NCL. |
[137a] |
|
35 |
Fmoc‐Cys(palmityl)‐OH |
N |
Incompatible with NCL. Fmoc‐D‐Cys(palmityl) must be synthesized. |
[137b] |
|
|
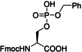
36 |
Tyr sulfation |
Fmoc‐Tyr(OTBS)−OH Deprotection and sulfation on‐resin with: |
N |
Accessibility of enantiomeric reagent. |
[131b] |
|
|
|
|
|
|
|
|
|
|
+DIEPA |
|
|
|
|
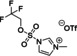
37 |
Ser phosphorylation |
|
Y |
Accessibility of enantiomeric reagent. |
[18, 130b, 159] |
|
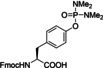
38 |
Tyrosine phosphorylation |
|
N |
Accessibility of enantiomeric reagent. |
[131a] |