Abstract
In Chlamydomonas reinhardtii y-1, newly synthesized chlorophyll a/b-binding apoproteins are degraded when chlorophylls are not present for assembly of stable light-harvesting complexes. A protease was purified from the membrane fraction of degreened y-1 cells, which digested chlorophyll a/b-binding proteins in membranes from C. reinhardtii pg-113, a protease-deficient strain. This protease was active with p-nitroanilides of nonpolar amino acids (Leu and Phe), but not of basic amino acids (Lys and Arg). The apparent molecular weight of the enzyme is 38,000 ± 2,000 as determined by electrophoresis in the presence of sodium dodecyl sulfate. Typical inhibitors of the major classes of proteases were ineffective with this enzyme. Protease activity was constant from pH 7.5 to 9; a plot of log V versus pH suggested that deprotonation of an ionizable group with a pK value of 6.0 to 6.5 is required for activity. The protease was inactivated by diethylpyrocarbonate and by photooxidation sensitized by rose bengal. These results suggested that a histidyl residue is required for catalysis. Although very sensitive to photodynamic conditions in vitro, the enzyme was not inactivated in vivo when cells were exposed to light.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Regulation of photosynthesis by reversible phosphorylation of the light-harvesting chlorophyll a/b protein. Biochem J. 1983 Apr 15;212(1):1–13. doi: 10.1042/bj2120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Girard V., Maclachlan G. Modulation of Pea Membrane beta-Glucan Synthase Activity by Calcium, Polycation, Endogenous Protease, and Protease Inhibitor. Plant Physiol. 1987 Sep;85(1):131–136. doi: 10.1104/pp.85.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin C., Elderfield P. D., James H. E., Zimmermann R., Dunbar B., Robinson C. The reaction specificities of the thylakoidal processing peptidase and Escherichia coli leader peptidase are identical. EMBO J. 1989 Dec 1;8(12):3917–3921. doi: 10.1002/j.1460-2075.1989.tb08572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
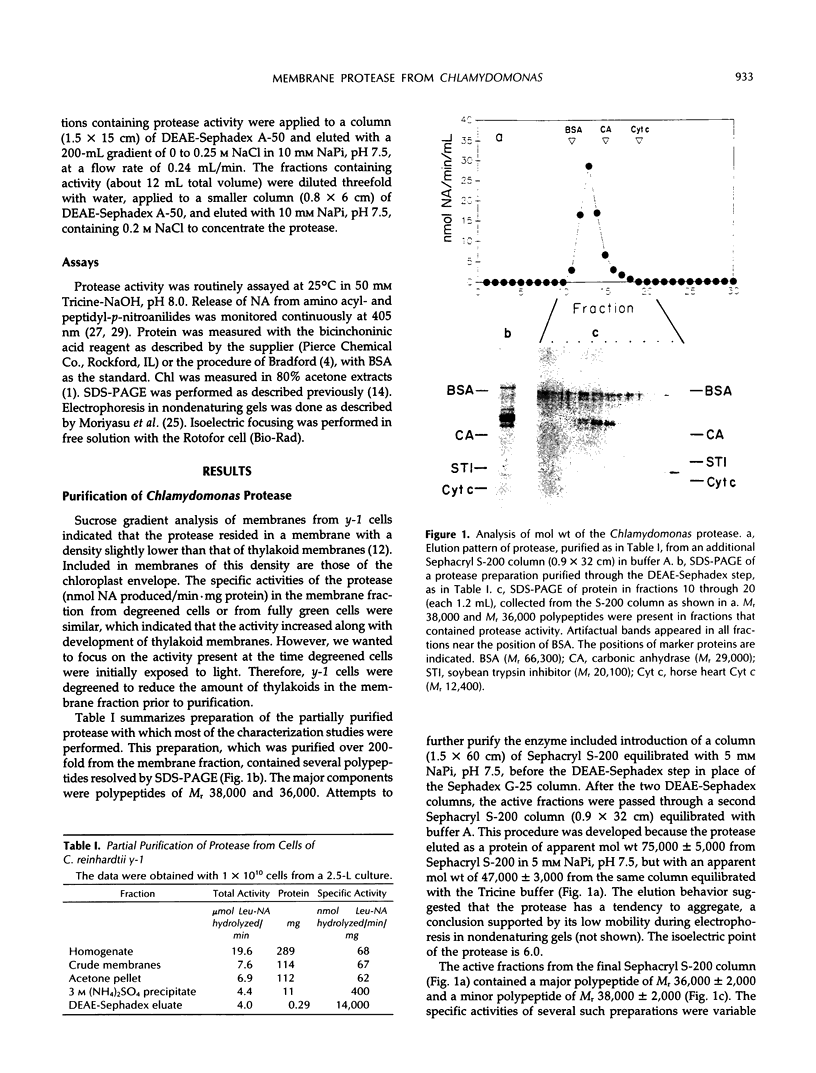
- Hoober J. K., Maloney M. A., Asbury L. R., Marks D. B. Accumulation of Chlorophyll a/b-Binding Polypeptides in Chlamydomonas reinhardtii y-1 in the Light or Dark at 38 degrees C : Evidence for Proteolytic Control. Plant Physiol. 1990 Feb;92(2):419–426. doi: 10.1104/pp.92.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Marks D. B., Keller B. J., Margulies M. M. Regulation of accumulation of the major thylakoid polypeptides in Chlamydomonas reinhardtii y-1 at 25 degrees C and 38 degrees C. J Cell Biol. 1982 Nov;95(2 Pt 1):552–558. doi: 10.1083/jcb.95.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
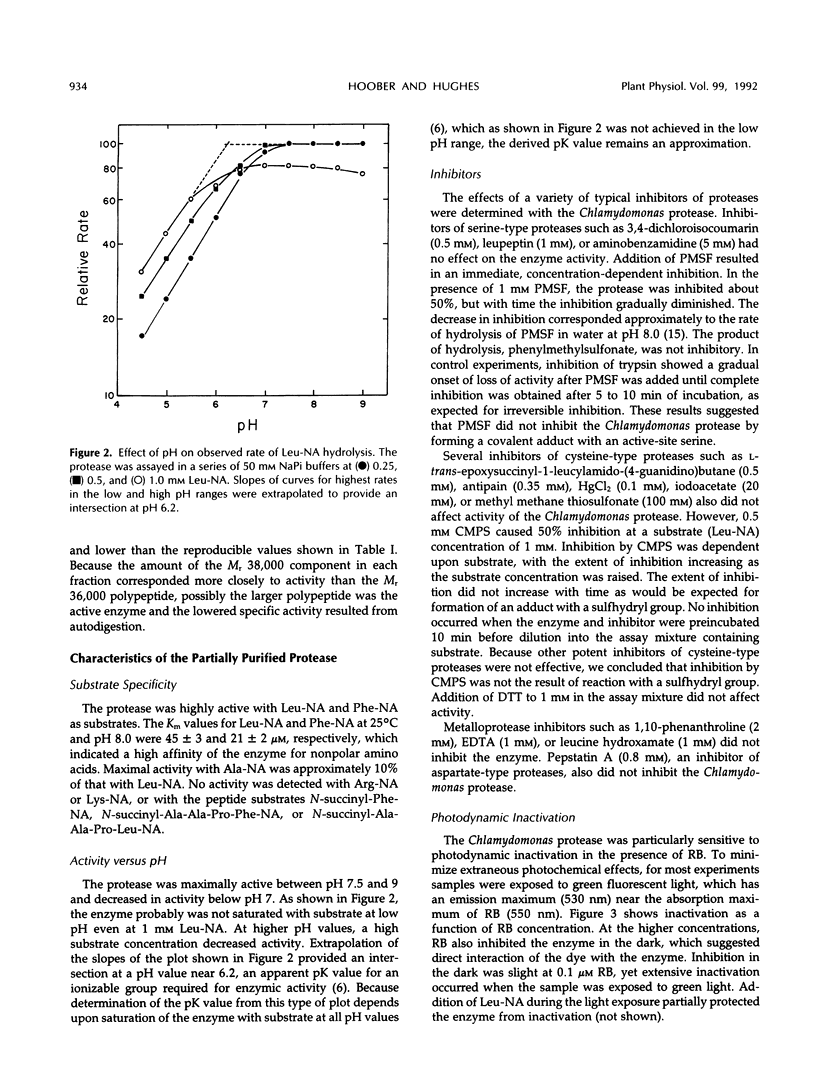
- Hoober J. K., Millington R. H., D'Angelo L. P. Structural similarities between the major polypeptides of thylakoid membranes from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1980 Jun;202(1):221–234. doi: 10.1016/0003-9861(80)90424-5. [DOI] [PubMed] [Google Scholar]
- James G. T. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Anal Biochem. 1978 Jun 1;86(2):574–579. doi: 10.1016/0003-2697(78)90784-4. [DOI] [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Robinson C. Transport of proteins into chloroplasts. Partial purification of a thylakoidal processing peptidase involved in plastocyanin biogenesis. J Biol Chem. 1987 Dec 5;262(34):16386–16390. [PubMed] [Google Scholar]
- Liu X. Q., Jagendorf A. T. Neutral peptidases in the stroma of pea chloroplasts. Plant Physiol. 1986 Jun;81(2):603–608. doi: 10.1104/pp.81.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney M. A., Hoober J. K., Marks D. B. Kinetics of Chlorophyll Accumulation and Formation of Chlorophyll-Protein Complexes during Greening of Chlamydomonas reinhardtii y-1 at 38 degrees C. Plant Physiol. 1989 Nov;91(3):1100–1106. doi: 10.1104/pp.91.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D. B., Keller B. J., Hoober J. K. In Vitro Processing of Precursors of Thylakoid Membrane Proteins of Chlamydomonas reinhardtii y-1. Plant Physiol. 1985 Sep;79(1):108–113. doi: 10.1104/pp.79.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Moriyasu Y., Sakano K., Tazawa M. Vacuolar/Extravacuolar Distribution of Aminopeptidases in Giant Alga Chara australis and Partial Purification of One Such Enzyme. Plant Physiol. 1987 Jul;84(3):720–725. doi: 10.1104/pp.84.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter G. F., Thornber J. P. Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J Biol Chem. 1991 Sep 5;266(25):16745–16754. [PubMed] [Google Scholar]
- TUPPY H., WIESBAUER U., WINTERSBERGER E. [Amino acid-p-nitroanilide as a substrate for aminopeptidases and other proteolytic enzymes]. Hoppe Seylers Z Physiol Chem. 1962 Nov 15;329:278–288. doi: 10.1515/bchm2.1962.329.1.278. [DOI] [PubMed] [Google Scholar]
- de Barros E. G., Larkins B. A. Purification and characterization of zein-degrading proteases from endosperm of germinating maize seeds. Plant Physiol. 1990 Sep;94(1):297–303. doi: 10.1104/pp.94.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]