Abstract
Physiological regulation of nodule gas permeability has a central role in the response of legumes to such diverse factors as drought, defoliation, and soil nitrate. A new method for quantifying nodule respiration and O2 permeability, based on noninvasive spectrophotometry of leghemoglobin, was evaluated using intact, attached nodules of Lotus corniculatus. First, the relationship between nodule respiration (O2 consumption) rate and internal O2 concentration was determined from the rate of decrease in fractional oxygenation of leghemoglobin (FOL) under N2. The rate of increase of FOL under 100% O2 was then used to calculate nodule O2 permeability, after correcting for respiration. Inactivation of nitrogenase by exposure to 100% O2 for 15 minutes led to decreases in both permeability and O2-saturated respiration (Vmax), but the brief (<15 seconds) exposures to 100% O2 required by the assay itself had little effect on either parameter. A gradual increase in external O2 concentration from 20 to 40% resulted in a reversible decrease in permeability, but no change in Vmax. The new method is likely to be useful for research on nodule physiology and might also be applicable to agronomic research and crop improvement programs.
Full text
PDF

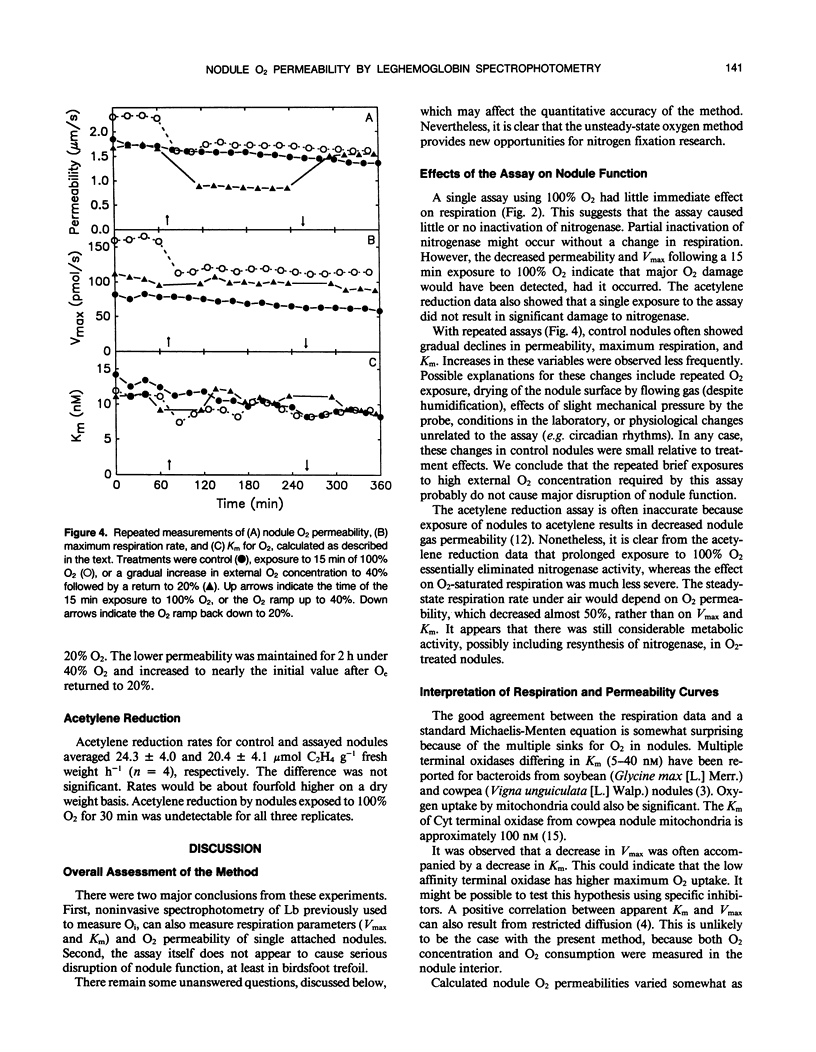


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denison R. F., Weisz P. R., Sinclair T. R. Analysis of acetylene reduction rates of soybean nodules at low acetylene concentrations. Plant Physiol. 1983 Nov;73(3):648–651. doi: 10.1104/pp.73.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S., King B. J., Canvin D. T., Layzell D. B. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987 May;84(1):164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Hunt S., Weagle G. E., Walsh K. B., Pottier R. H., Canvin D. T., Layzell D. B. Regulation of o(2) concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988 Jun;87(2):296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. D., Larue T. A. Effects of carbohydrate on the internal oxygen concentration, oxygen uptake, and nitrogenase activity in detached pea nodules. Plant Physiol. 1989 Oct;91(2):603–609. doi: 10.1104/pp.91.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawsthorne S., Larue T. A. Metabolism under Microaerobic Conditions of Mitochondria from Cowpea Nodules. Plant Physiol. 1986 Aug;81(4):1097–1102. doi: 10.1104/pp.81.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair T. R., Goudriaan J. Physical and morphological constraints on transport in nodules. Plant Physiol. 1981 Jan;67(1):143–145. doi: 10.1104/pp.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Denison R. F., Sinclair T. R. Response to drought stress of nitrogen fixation (acetylene reduction) rates by field-grown soybeans. Plant Physiol. 1985 Jul;78(3):525–530. doi: 10.1104/pp.78.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Sinclair T. R. Regulation of Soybean Nitrogen Fixation in Response to Rhizosphere Oxygen: II. Quantification of Nodule Gas Permeability. Plant Physiol. 1987 Jul;84(3):906–910. doi: 10.1104/pp.84.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]