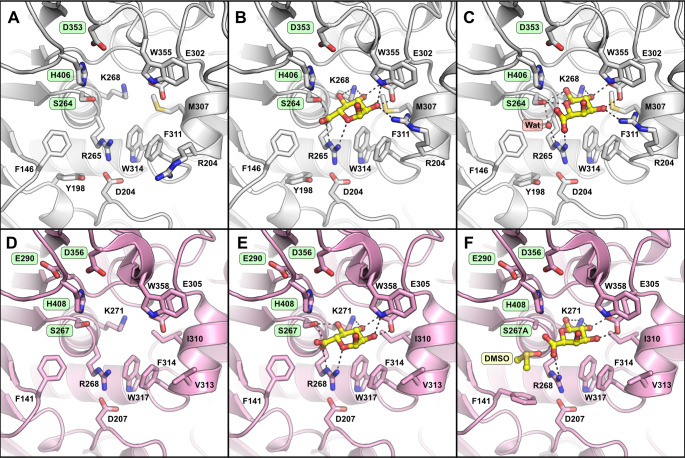
Fig 5.
Active site organization of PvCE15 in complex with uronate substrates. The active site of PvCE15 (A, PDB accession: 8Q6S) in complex with glucuronate (B, PDB accession: 8QCL), and galacturonate (C, PDB accession: 8QEF) compared to the active site of OtCE15A (D, PDB accession: 6GS0) in complex with glucuronate (E, PDB accession: 6SYR) and galacturonate (F, PDB accession: 6SZO). Residues of the catalytic triad and the additional catalytic acidic residue in OtCE15A are highlighted in green, and the water and DMSO molecules found in the galacturonate complex structures are highlighted in red and yellow, respectively. The galacturonate complex with OtCE15A was obtained with the catalytic serine substitution (S267A). Key interaction distances ≤3 Å are shown as black dashes.