Abstract
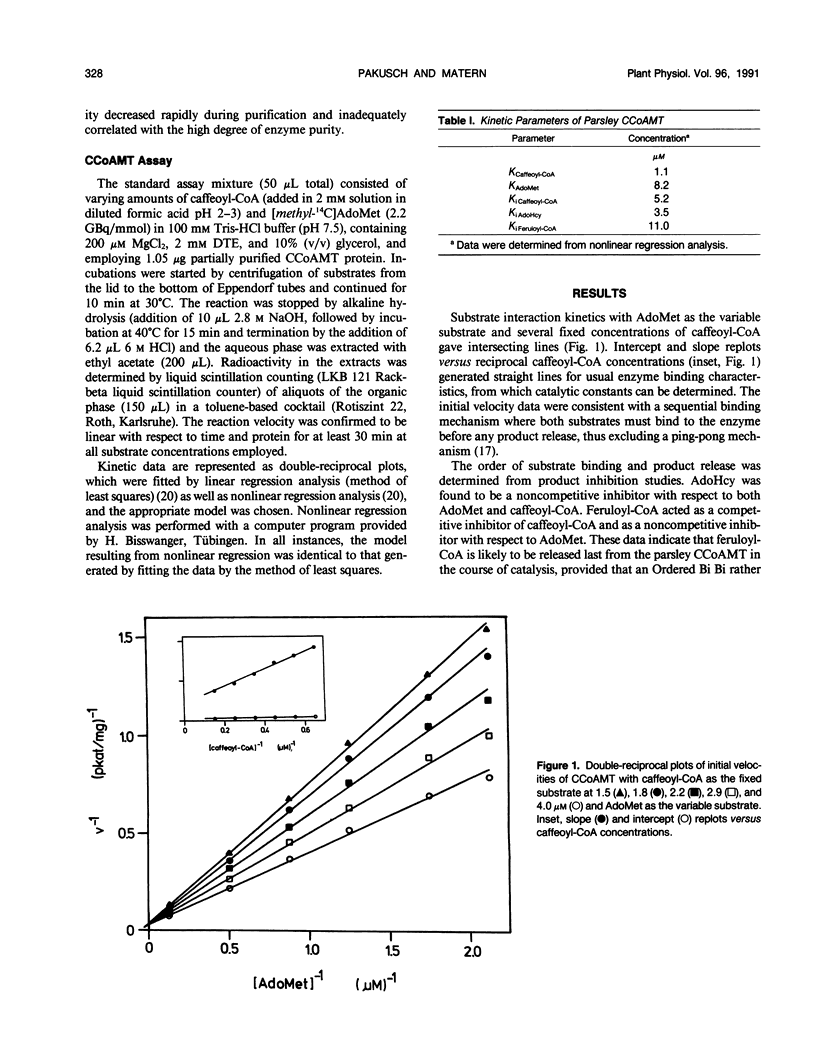
The activity of caffeoyl-coenzyme A (CoA) 3-O-methyltransferase, an enzyme widely distributed in plants and involved in cell wall reinforcement in a disease resistance response, appears to be subject to a complex type of regulation in vivo. In cultured parsley (Petroselinum crispum) cells treated with an elicitor from Phytophthora megasperma f.sp. glycinea, the enzyme activity is rapidly induced by a transient increase in the rate of de novo transcription. Parsley caffeoyl-CoA-specific methyltransferase differs in several aspects from other plant O-methyltransferases but shows limited homology to bacterial adenine-specific DNA methyltransferases. Kinetic analysis revealed an Ordered Bi Bi mechanism for catalysis, with caffeoyl-CoA bound prior to S-adenosyl-l-methionine and feruloyl-CoA released last from the enzyme. The small inhibitory constant determined in vitro for feruloyl-CoA suggests that, in vivo, the enzyme activity is also under tight control by the steady-state product concentration in addition to the rate of transcription that becomes affected upon elicitor challenge.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- De Luca V., Ibrahim R. K. Enzymatic synthesis of polymethylated flavonols in Chrysosplenium americanum. II. Substrate interaction and product inhibition studies of flavonol 3-, 6-, and 4'-O-methyltransferases. Arch Biochem Biophys. 1985 May 1;238(2):606–618. doi: 10.1016/0003-9861(85)90206-1. [DOI] [PubMed] [Google Scholar]
- Flohe L., Schwabe K. P. Kinetics of purified catechol O-methyltransferase. Biochim Biophys Acta. 1970 Dec 16;220(3):469–476. doi: 10.1016/0005-2744(70)90278-0. [DOI] [PubMed] [Google Scholar]
- Hermann C., Legrand M., Geoffroy P., Fritig B. Enzymatic synthesis of lignin: purification to homogeneity of the three O-methyltransferases of tobacco and production of specific antibodies. Arch Biochem Biophys. 1987 Mar;253(2):367–376. doi: 10.1016/0003-9861(87)90190-1. [DOI] [PubMed] [Google Scholar]
- Ingrosso D., Fowler A. V., Bleibaum J., Clarke S. Sequence of the D-aspartyl/L-isoaspartyl protein methyltransferase from human erythrocytes. Common sequence motifs for protein, DNA, RNA, and small molecule S-adenosylmethionine-dependent methyltransferases. J Biol Chem. 1989 Nov 25;264(33):20131–20139. [PubMed] [Google Scholar]
- Khouri H. E., Tahara S., Ibrahim R. K. Partial purification, characterization, and kinetic analysis of isoflavone 5-O-methyltransferase from yellow lupin roots. Arch Biochem Biophys. 1988 May 1;262(2):592–598. doi: 10.1016/0003-9861(88)90410-9. [DOI] [PubMed] [Google Scholar]
- Knogge W., Weissenböck G. Purification, characterization, and kinetic mechanism of S-adenosyl-L-methionine: vitexin 2"-O-rhamnoside 7-O-methyltransferase of Avena sativa L. Eur J Biochem. 1984 Apr 2;140(1):113–118. doi: 10.1111/j.1432-1033.1984.tb08073.x. [DOI] [PubMed] [Google Scholar]
- Lauster R. Evolution of type II DNA methyltransferases. A gene duplication model. J Mol Biol. 1989 Mar 20;206(2):313–321. doi: 10.1016/0022-2836(89)90481-6. [DOI] [PubMed] [Google Scholar]
- Pakusch A. E., Matern U., Schiltz E. Elicitor-Inducible Caffeoyl-Coenzyme A 3-O-Methyltransferase from Petroselinum crispum Cell Suspensions : Purification, Partial Sequence, and Antigenicity. Plant Physiol. 1991 Jan;95(1):137–143. doi: 10.1104/pp.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckigt J., Zenk M. H. Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives. Z Naturforsch C. 1975 May-Jun;30(3):352–358. doi: 10.1515/znc-1975-5-609. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee W. C., Eglsaer S. J., Richards W. R. Confirmation of a ping-pong mechanism for S-adenosyl-L-methionine:magnesium protoporphyrin methyltransferase of etiolated wheat by an exchange reaction. Biochem Biophys Res Commun. 1989 Jul 14;162(1):483–490. doi: 10.1016/0006-291x(89)92023-8. [DOI] [PubMed] [Google Scholar]


