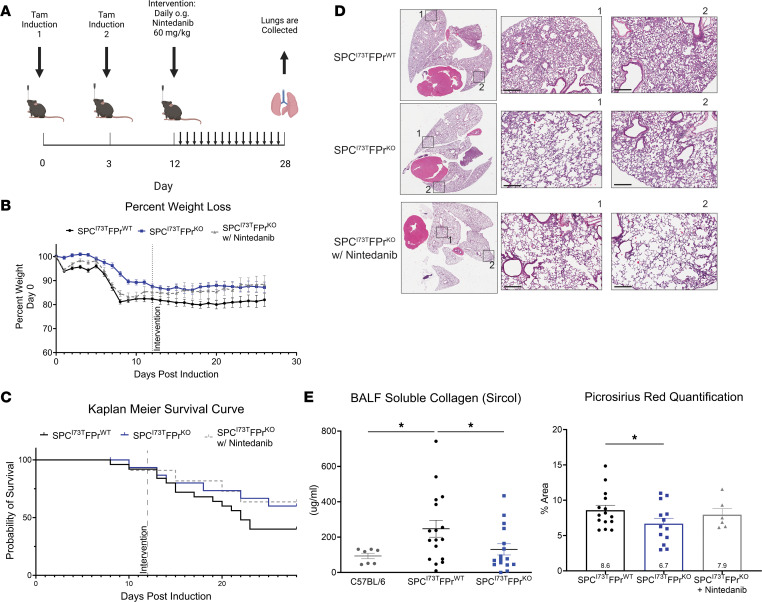
Figure 3. Nintedanib intervention is not additive to Ptgfr deficiency in IER-SftpcI73T mice.
(A) Daily nintedanib intervention (60 mg/kg) was initiated at D12 after TAM induction. Following 16 days of intervention, surviving mice were euthanized and processed to evaluate fibrotic endpoints. (B) Weight loss as a percent of starting weight was tracked throughout the study; nintedanib intervention in IER-SftpcI73T/Ptgfr–/– mice did not reduce mean weight loss. (C) Kaplan-Meier survival analysis by log-rank testing demonstrates a nonsignificant improved probability of survival in IER-SftpcI73T/Ptgfr–/– that was not improved through nintedanib intervention. (D) Representative histology from IER-SftpcI73T/Ptgfr+/+, IER-SftpcI73T/Ptgfr–/–, and nintedanib-treated IER-SftpcI73T/Ptgfr–/– mice 28 days after TAM and development of fibrosis. Images are derived from H&E-stained sections. Scale bars: 300 μM. (E) Soluble collagen in BALF as measured by Sircol assay and fibrillar collagen in histological sections measured by PSR staining demonstrated a significant decrease in IER-SftpcI73T/Ptgfr–/– mice; again, nintedanib treatment did not improve these outcomes. Quantification of PSR was performed using ImageJ, and data represent percentage of total section area. Survival and weight loss data are derived from IER-SftpcI73T/Ptgfr+/+ (n = 26), IER-SftpcI73T/Ptgfr–/– without nintedanib (n = 12), and IER-SftpcI73T/Ptgfr–/– with nintedanib (n = 12). Soluble collagen and PSR analysis included IER-SftpcI73T/Ptgfr+/+ (n = 14), IER-SftpcI73T/Ptgfr–/– without nintedanib (n = 11), and IER-SftpcI73T/Ptgfr–/– with nintedanib (n = 7). Ordinary 1-way ANOVA testing was performed. *P < 0.05.