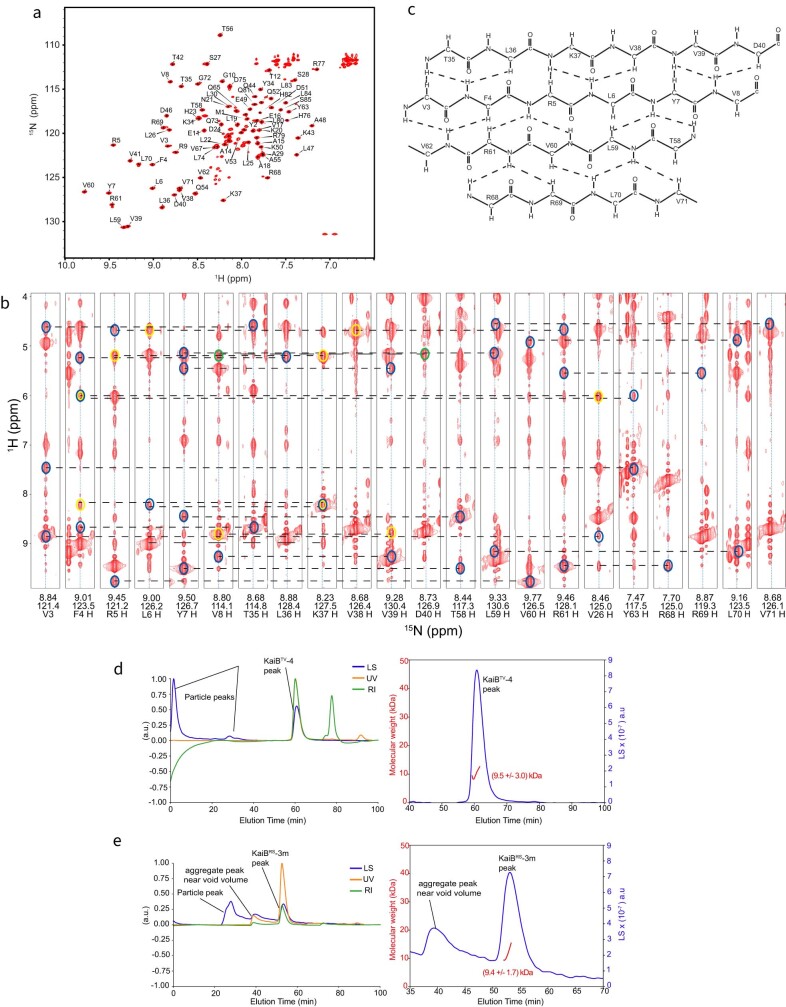
Extended Data Fig. 4. Supplemental experimental data for KaiBTV-4 and KaiBRS-3m.
a) 1H 15N HSQC spectra of KaiBTV-4 indicates one major folded state. Assignments are shown. b) Strip plot extracted from a 150 ms mixing time 15N-edited NOESY-HSQC spectrum of KaiBTV-4 illustrating the inter-strand NOEs between residues V3-V8; T35-D40; T58-Y63; R68-Y71, used in confirming KaiBTV-4 is in the fold-switched state. c) Summary of NOEs between the parallel β-sheets V3-V8 and T35-D40, and the antiparallel β-sheets T58-V62 and R68-V71. Confirmed NOEs are depicted by dashed lines. NOEs not depicted could not be confirmed unambiguously. SEC-MALS analysis of (d) KaiBTV-4 and (e) KaiBRS-3m at NMR concentration of 500 μM indicate both are monomeric. The profiles on the left show the full SEC-MALS run with the light scattering (LS) profile in blue, normalized UV profile in red, and refractive index (RI) profile in green. On the right is the region of the peak of interest showing the light scattering profile (blue) plotted against elution time, and the protein molar masses are indicated in red. The molar masses of KaiBTV−4 and KaiBRS-3m have been determined from light scattering and refractometry data to be (9.5 +/− 3.0) kDa and (9.4 +/− 1.7) kDa, respectively.