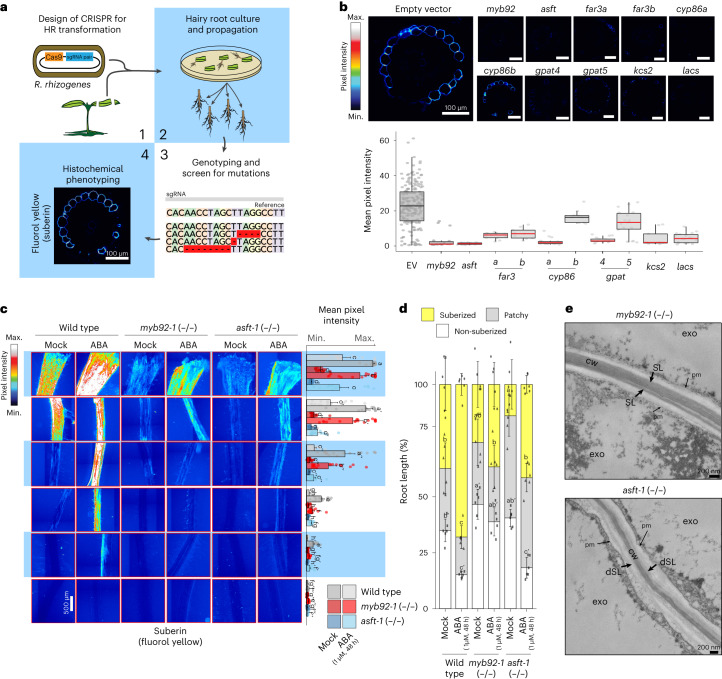
Fig. 3. Loss-of-function mutant alleles of candidate genes disrupt exodermal suberization in tomato.
a, Graphical summary of the hairy root (HR; R. rhizogenes) mutant screen. b, Summary of mutant phenotypes of candidate genes in hairy roots. Top: representative cross-sections of mature portions of the roots stained with fluorol yellow. Bottom: overall quantification of the fluorol yellow signal across multiple cross-sections (wild-type n = 66; rest n = 6). Red line indicates statistically significant differences in fluorol yellow pixel intensity in the mutant versus wild type as determined with a one-way ANOVA followed by a Tukey–Kramer post hoc test (Padj < 0.05); EV, empty vector. Box plot centres depict the median while the bottom and top box limits depict the 25th and 75th percentile, respectively. Whiskers represent minima and maxima. Dots depict individual samples. c, Fluorol yellow staining for suberin in 7-day-old wild-type (repeated from Fig. 1 for reference), slmyb92-1 and slasft-1 plants treated with mock or 1 µM ABA for 48 h. Whole-mount staining of primary roots across different sections (left) and mean intensity of fluorol yellow signal along the root (right) (n = 6). Letters indicate significant differences (one-way ANOVA followed by a Tukey–Kramer post hoc test (Padj < 0.05). Error bars, s.d. d, Developmental stages of suberin deposition in the 7-day-old wild-type and mutant plants treated with mock or 1 µM ABA for 48 h. Zones were classified as non-suberized (white), patchy suberized (grey) and continuously suberized (yellow) (n = 6). Letters indicate statistically different groups; apostrophes indicate different statistical comparisons e, Representative transmission electron microscopy cross-sections of slasft-1 and slmyb92-1 mutants obtained at 1 mm from the root–hypocotyl junction. The slasft-1 mutant presents a deficit in suberin lamellar structure. cw, cell wall; dSL, defective suberin lamellae; exo, exodermis; pm, plasma membrane; SL, suberin lamellae.