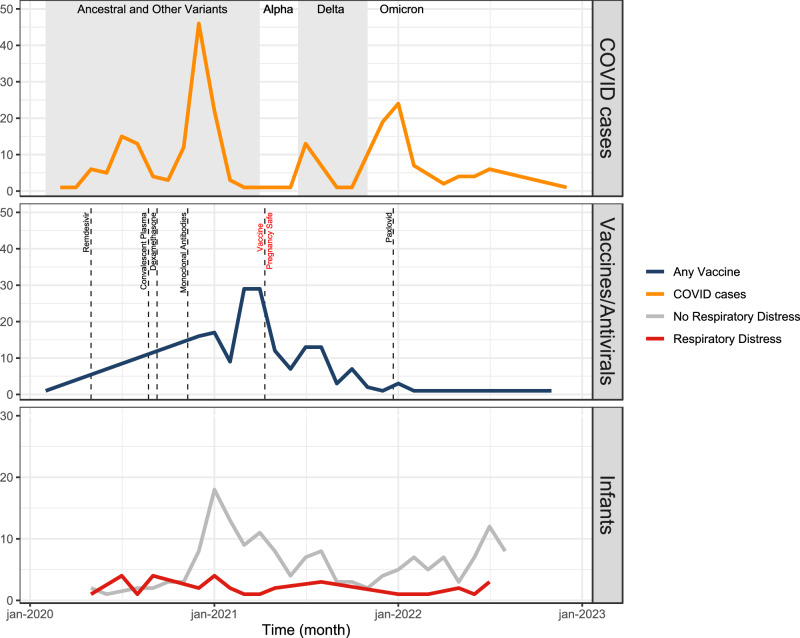
Fig. 2. Maternal COVID cases (n = 221), maternal immunizations (n = 70), and cases of infant respiratory distress (n = 34) overtime.
A COVID Cases: The gray shaded regions represent when different variants were circulating in California. Ancesteral and other variants circulated from 1 Feburary 2020 to 1 April 2021; the Alpha variant circulated from 1 April 2021 to 15 June 2021; the Delta variant circulated from 15 June 2021 to 15 December 2021; and the Omicron variant circulated from 15 December 2021 onward. B Immunizations: Dashed vertical lines signify key changes in treatment availability. On 1 May 2020 the FDA issued an emergency use authorization (EUA) for Remdesivir, on 23 August 2020 the FDA issued an EUA for convalescent plasma, on 3 September 2020 the Journal of the American Medical Association and the WHO recommended use of dexamethasone, on 9 November 2020 the FDA issued an EUA for use of monoclonal antibodies, on 11 August 2021 the CDC confirmed that vaccines were safe during pregnancy, and on 22 December 21 the FDA authorized Paxlovid use33. C Infant respiratory distress over time with red representing infants born with respiratory distress and gray representing infants born without respiratory distress.