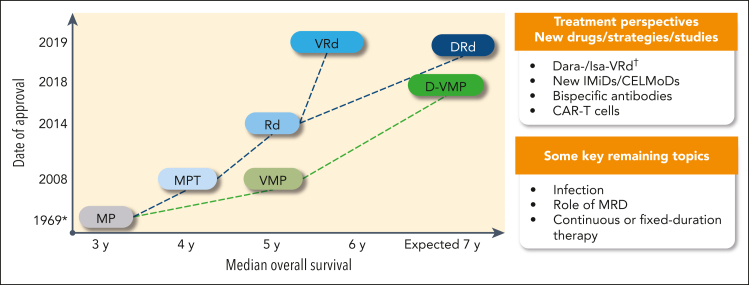
Figure 1.
Treatment landscape and perspective in newly diagnosed transplant-ineligible patients: regimens, date of approval (European Medicines Agency), and OS. ∗ indicates the publication date, not an approval date; †, #NCT03319667 and #NCT03652064. CAR-T, chimeric antigen receptor T cell; CELMoDs, cereblon E3 ligase modulation drugs; Dara, daratumumab; IMiD, immunomodulatory drug; Isa, isatuximab; MRD, minimal residual disease.