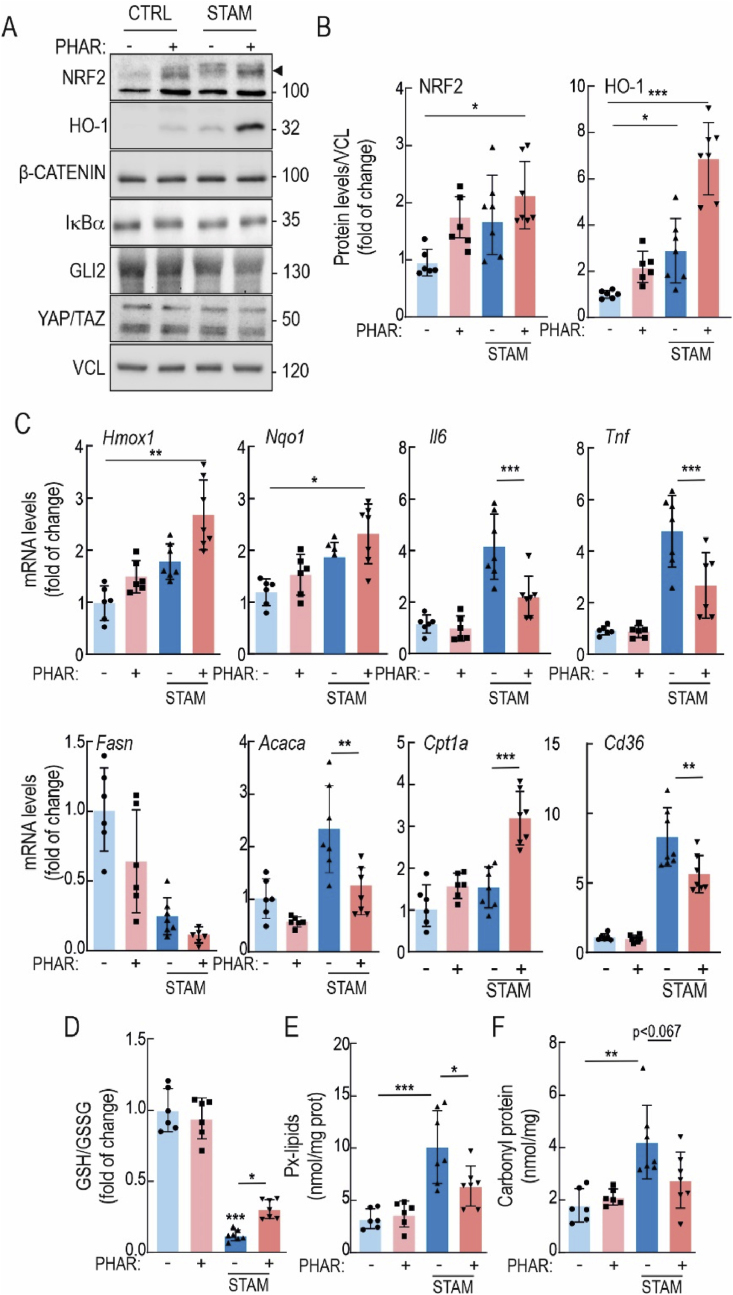
Fig. 2.
PHAR activates NRF2 in liver and protects against oxidative stress and inflammation in the STAM model of NASH. During weeks 8–10, mice in either the control or STAM groups were administered PHAR at a dose of 50 mg/kg/day. A, representative immunoblots of NRF2, HO-1, β-CATENIN, IĸBα, GLI2, YAP/TAZ, and VCL as a loading control. Black arrow indicates NRF2 specific band. B, densitometric analysis of NRF2 and HO-1 protein levels from representative immunoblots from A, expressed as a ratio of VCL. Data are mean ± S.D. (n = 6–7). *p < 0.05; ***p < 0,001 vs. CTRL-VEH according to a two-way ANOVA test followed by Bonferroni post-hoc test. C, mRNA levels of NRF2 targets Hmox1, and Nqo1, inflammatory markers Il6 and Tnf, lipid metabolic markers Fasn, Acaca, Cpt1a and Cd36, were determined by qRT-PCR and normalized by the geometric mean of Gapdh, Tbp, and Actb levels. Data are mean ± S.D. (n = 6–7). *p < 0,5; **p < 0,01; ***p < 0.001 vs. CTRL-VEH or STAM-VEH according to a two-way ANOVA followed by Bonferroni post-hoc test. D, levels of reduced glutathione (GSH) normalized with total oxidized glutathione (GSSG). E, Peroxidized lipids (Px-lipids) representing mostly MDA. F, protein carbonyl content as determined by DNPH levels. Data are mean ± S.D. (n = 6–7). *p < 0.05; **p < 0.01; ***p < 0.001 vs. CTRL-VEH or STAM-VEH according to a two-way ANOVA followed by Bonferroni post-hoc test.