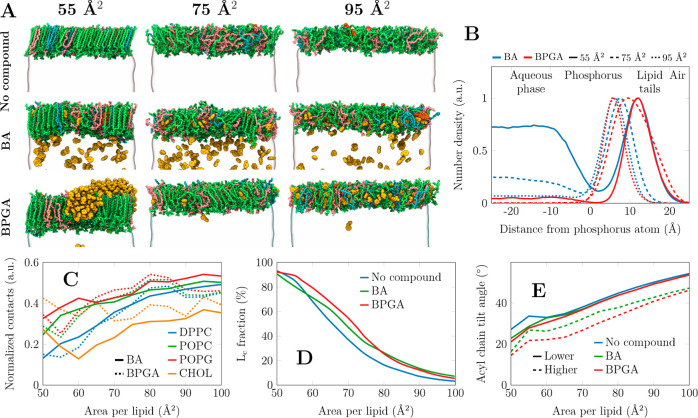
Figure 2.
Partition preferences of the vaping chemicals into the SLS monolayer from atomistic molecular dynamics simulations. (A) SLS monolayers composed of 149 lipids per monolayer, containing no vaping chemicals (top row), 320 benzaldehyde (BA) molecules (middle row), or 200 benzaldehyde propylene glycol acetal (BPGA) molecules (bottom row). The partitioning tendency at 10-fold smaller compound concentrations was similar (Figure S6), yet there were not enough vaping chemical molecules for aggregation. The final structures of simulations at three areas per lipid are shown. DPPC, POPC, POPG, and cholesterol are depicted in green, pink, cyan, and orange, respectively, whereas the vaping compounds are shown in yellow. Water is shown as a transparent surface, and all hydrogens are omitted for clarity. (B) The density profiles of BA and BPGA were across the lipid monolayer at the air–water interface. Data are shown at three compression states also visualized on the left side of the figure. The density profiles are normalized so that their maxima are set to 1. (C) Interaction preference of BA and BPGA with different lipids. As the lipid moieties are present in different amounts, the contacts are normalized by the number of possible interactions. (D) Fraction of acyl chains and cholesterol molecules that are tightly packed, hence resembling the Lc phase. (E) The tilt angle of the phospholipid acyl chains was obtained with no vaping chemicals as well as with lower and higher concentrations of either BA or BPGA.