Abstract
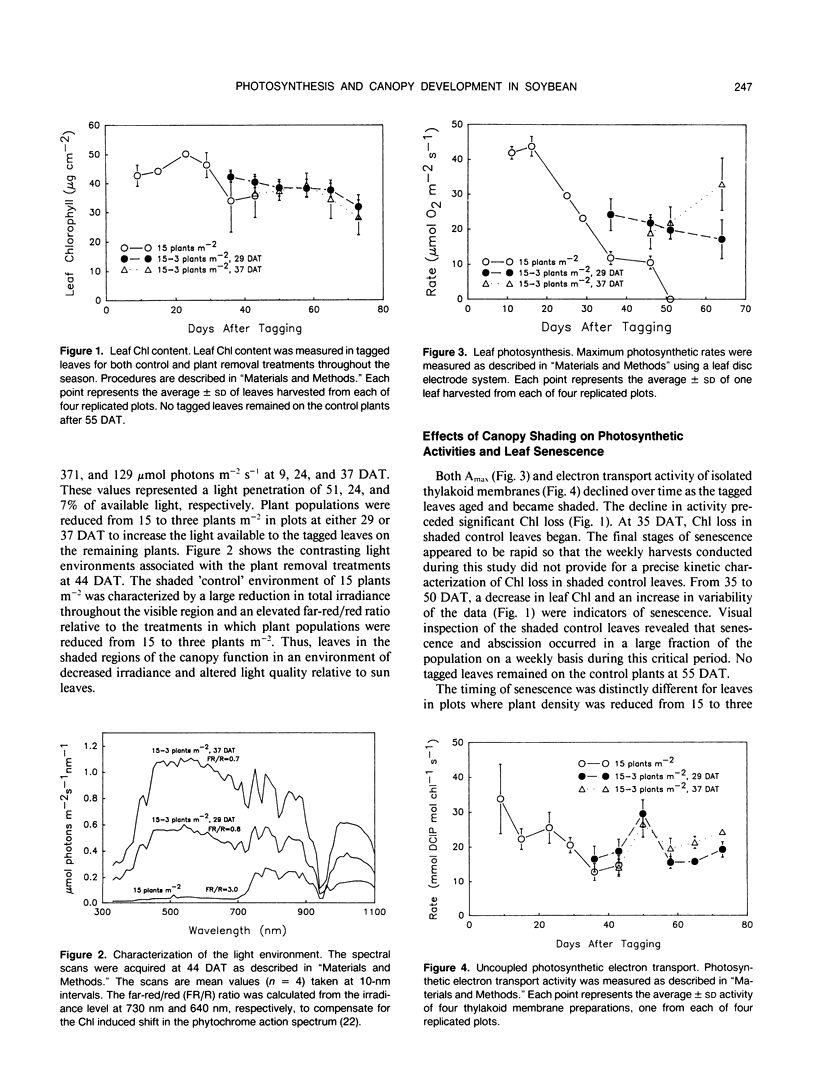
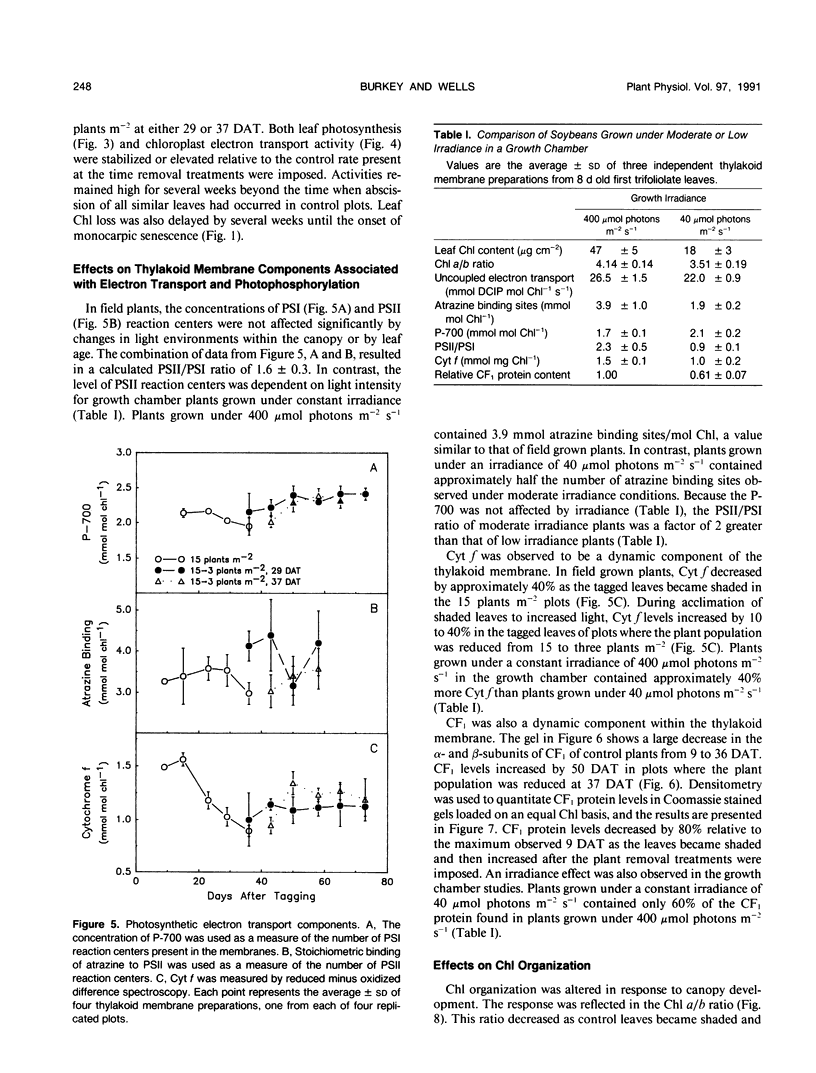
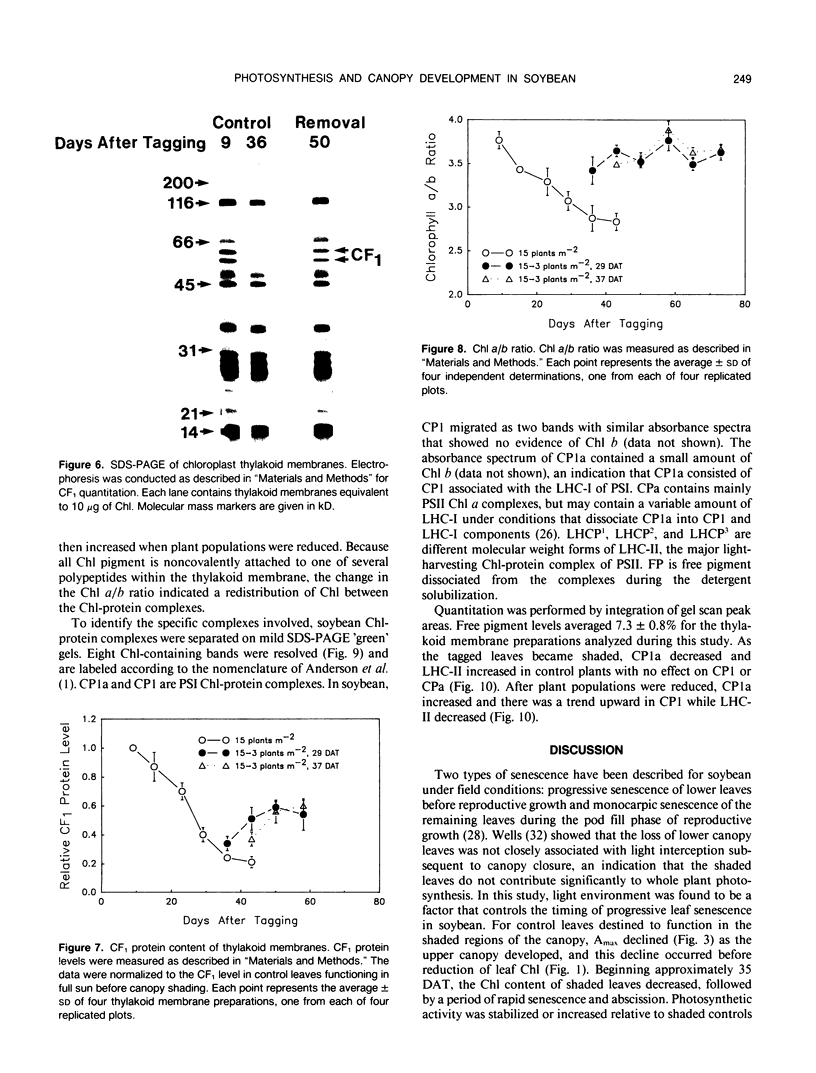
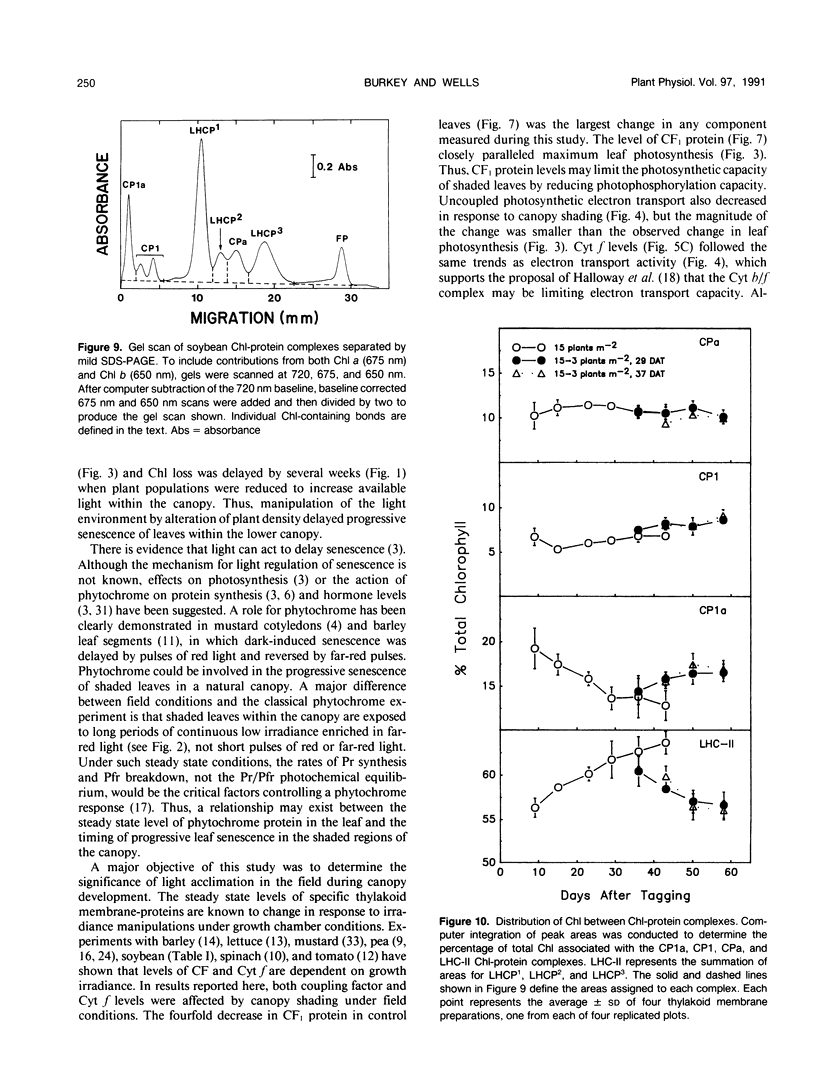
The effect of natural shading on photosynthetic capacity and chloroplast thylakoid membrane function was examined in soybean (Glycine max. cv Young) under field conditions using a randomized complete block design. Seedlings were thinned to 15 plants per square meter at 20 days after planting. Leaves destined to function in the shaded regions of the canopy were tagged during early expansion at 40 days after planting. To investigate the response of shaded leaves to an increase in available light, plants were removed from certain plots at 29 or 37 days after tagging to reduce the population from 15 to three plants per square meter and alter the irradiance and spectral quality of light. During the transition from a sun to a shade environment, maximum photosynthesis and chloroplast electron transport of control leaves decreased by two- to threefold over a period of 40 days followed by rapid senescence and abscission. Senescence and abscission of tagged leaves were delayed by more than 4 weeks in plots where plant populations were reduced to three plants per square meter. Maximum photosynthesis and chloroplast electron transport activity were stabilized or elevated in response to increased light when plant populations were reduced from 15 to three plants per square meter. Several chloroplast thylakoid membrane components were affected by light environment. Cytochrome f and coupling factor protein decreased by 40% and 80%, respectively, as control leaves became shaded and then increased when shaded leaves acclimated to high light. The concentrations of photosystem I (PSI) and photosystem II (PSII) reaction centers were not affected by light environment or leaf age in field grown plants, resulting in a constant PSII/PSI ratio of 1.6 ± 0.3. Analysis of the chlorophyll-protein composition revealed a shift in chlorophyll from PSI to PSII as leaves became shaded and a reversal of this process when shaded leaves were provided with increased light. These results were in contrast to those of soybeans grown in a growth chamber where the PSII/PSI ratio as well as cytochrome f and coupling factor protein levels were dependent on growth irradiance. To summarize, light environment regulated both the photosynthetic characteristics and the timing of senescence in soybean leaves grown under field conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chow W. S., Melis A., Anderson J. M. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7502–7506. doi: 10.1073/pnas.87.19.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Holloway P. J., Maclean D. J., Scott K. J. Rate-Limiting Steps of Electron Transport in Chloroplasts during Ontogeny and Senescence of Barley. Plant Physiol. 1983 Jul;72(3):795–801. doi: 10.1104/pp.72.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Kasperbauer M. J. Far-Red Light Reflection from Green Leaves and Effects on Phytochrome-Mediated Assimilate Partitioning under Field Conditions. Plant Physiol. 1987 Oct;85(2):350–354. doi: 10.1104/pp.85.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. J., Whitmarsh J. Photosynthetic apparatus of pea thylakoid membranes : response to growth light intensity. Plant Physiol. 1989 Mar;89(3):932–940. doi: 10.1104/pp.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J. G., Krueger R. W., Miles D. Chlorophyll-Protein Complexes of a Photosystem II Mutant of Maize : Evidence that Chlorophyll-Protein a-2 and a Chlorophyll-Protein Complex Derived from a Photosystem I Antennae System Comigrate on Polyacrylamide Gels. Plant Physiol. 1984 May;75(1):238–241. doi: 10.1104/pp.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with n,n-dimethylformamide. Plant Physiol. 1982 Jun;69(6):1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biophys Acta. 1977 Apr 11;460(1):113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]



