Abstract
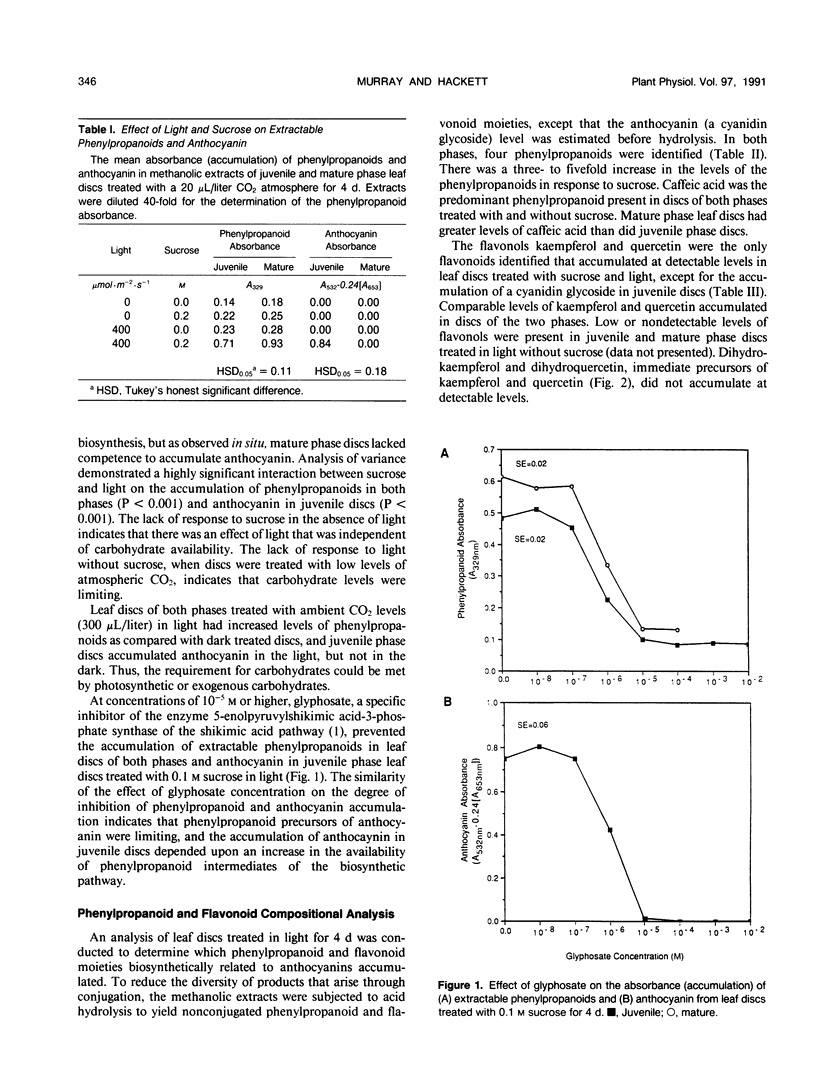
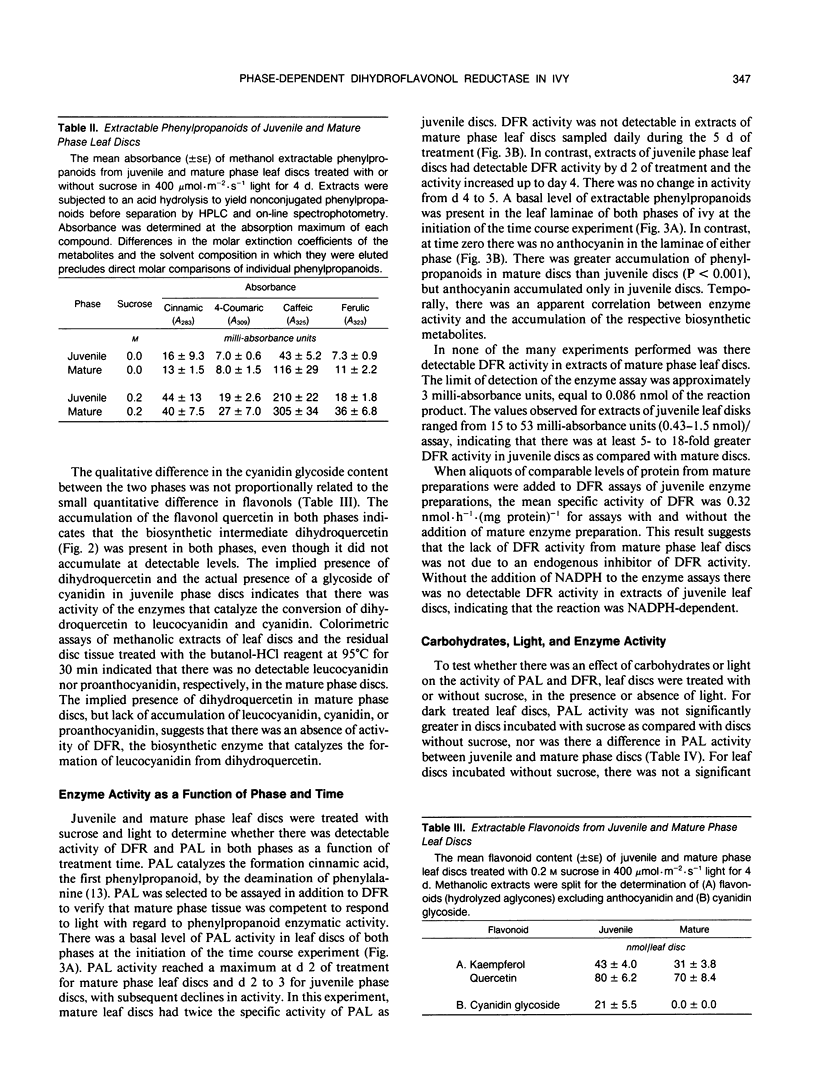
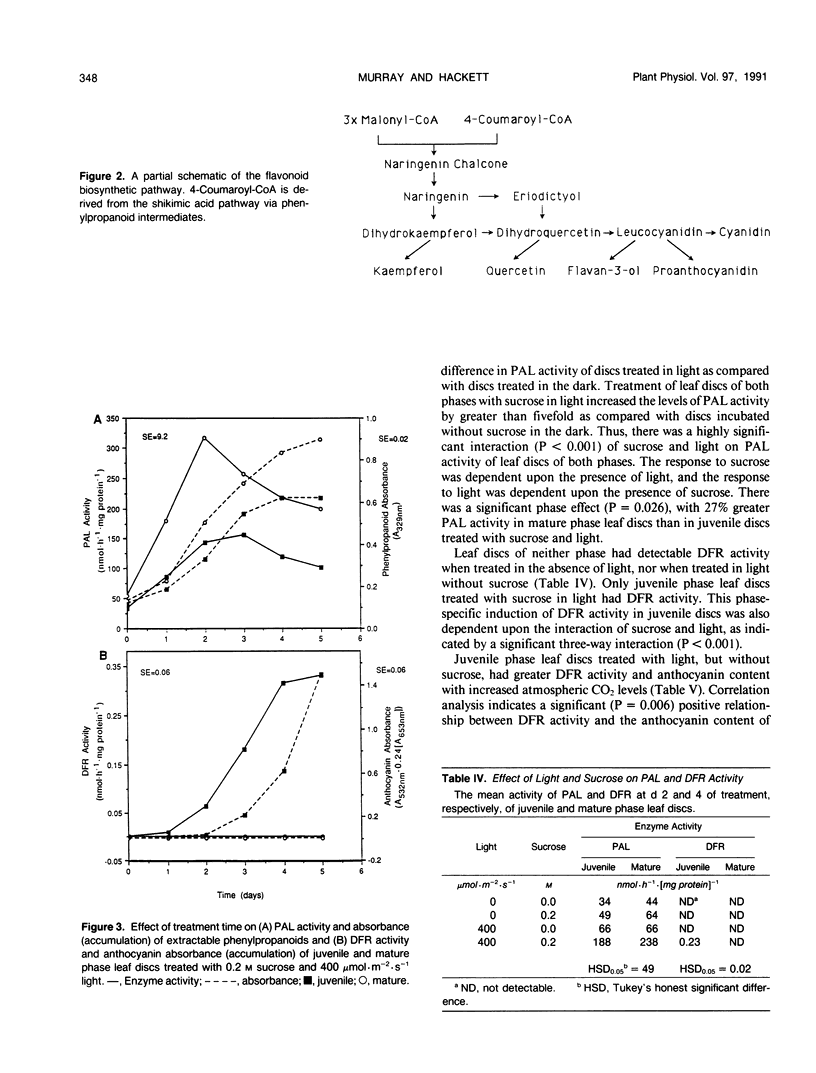
Juvenile phase English ivy (Hedera helix L.) plants accumulate anthocyanin pigment in the hypodermis of stems and petioles, whereas genetically identical plants of the mature phase do not. The objective of this work was to assess which enzyme(s) might limit anthocyanin accumulation in mature phase ivy. Leaf discs of both juvenile and mature phase ivy accumulated comparable levels of the flavonols kaempferol and quercetin, whereas only juvenile phase discs accumulated anthocyanin. The accumulation of quercetin, but lack of accumulation of leucocyanidin or anthocyanin in mature phase discs, suggested that mature discs lacked dihydroflavonol reductase activity. There was no detectable dihydroflavonol reductase activity in mature phase discs, whereas there was an induction of activity in juvenile phase discs in response to sucrose, or photosynthetically fixed carbon, and light as a photomorphogenic signal. Phenylalanine ammonia-lyase, an enzyme early in the anthocyanin biosynthetic pathway, was induced above its basal level by sucrose and light in discs of both phases of ivy, with greater activity in mature phase discs. Phenylpropanoids, a class of compounds that are precursors to flavonoids, accumulated in leaf discs of both phases, with greater levels in mature phase discs. These results indicate that the lack of dihydroflavonol reductase activity limits the accumulation of anthocyanin in mature phase tissue.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ebel J., Hahlbrock K. Enzymes of flavone and flavonol-glycoside biosynthesis. Coordinated and selective induction in cell-suspension cultures of Petroselinum hortense. Eur J Biochem. 1977 May 2;75(1):201–209. doi: 10.1111/j.1432-1033.1977.tb11518.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Knobloch K. H., Kreuzaler F., Potts J. R., Wellmann E. Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1976 Jan 2;61(1):199–206. doi: 10.1111/j.1432-1033.1976.tb10012.x. [DOI] [PubMed] [Google Scholar]
- Stafford H. A., Lester H. H. Enzymic and nonenzymic reduction of (+)-dihydroquercetin to its 3,4,-diol. Plant Physiol. 1982 Sep;70(3):695–698. doi: 10.1104/pp.70.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A., Lester H. H. Flavan-3-ol Biosynthesis : The Conversion of (+)-Dihydroquercetin and Flavan-3,4-cis-Diol (Leucocyanidin) to (+)-Catechin by Reductases Extracted from Cell Suspension Cultures of Douglas Fir. Plant Physiol. 1984 Sep;76(1):184–186. doi: 10.1104/pp.76.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Sherwood R. T. Regulation of lignin formation in reed canarygrass in relation to disease resistance. Plant Physiol. 1976 Jun;57(6):915–919. doi: 10.1104/pp.57.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]


