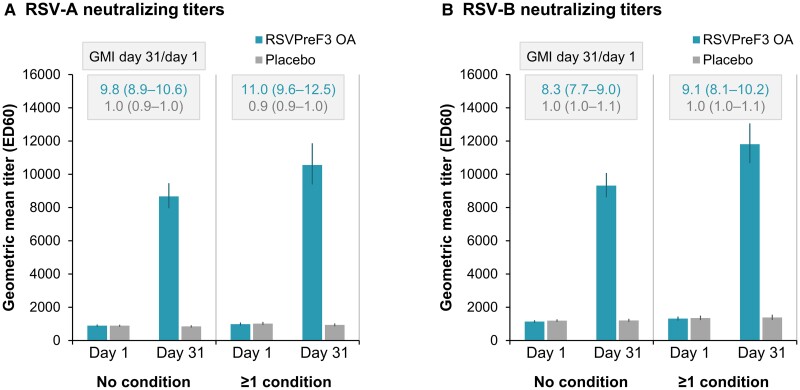
Figure 2.
RSV-A and RSV-B neutralizing titers before and 1 month after RSVPreF3 OA or placebo administration, by coexisting medical conditions of interest (per-protocol population for immunogenicity). Graphs show respiratory syncytial virus (RSV) subtypes A and B neutralizing titers before (day 1) and 1 month after (day 31) administration of the AS01E-adjuvanted RSV prefusion F protein–based vaccine (RSVPreF3 OA) or placebo for the subgroups of participants without any of the coexisting medical conditions of interest (no condition) or with at least 1 of these conditions (≥1 condition); conditions of interest were cardiorespiratory conditions (chronic obstructive pulmonary disease [COPD], asthma, any chronic respiratory or pulmonary disease [including COPD, asthma, and other conditions], chronic heart failure) and endocrine and metabolic conditions (diabetes mellitus type 1 or type 2 and advanced liver or renal disease) that are associated with an increased risk of severe RSV disease. Error bars depict 95% confidence intervals. Rectangles above the bars contain geometric mean increases (GMIs) from day 1 to day 31 with 95% confidence intervals. ED60, estimated dilution 60.