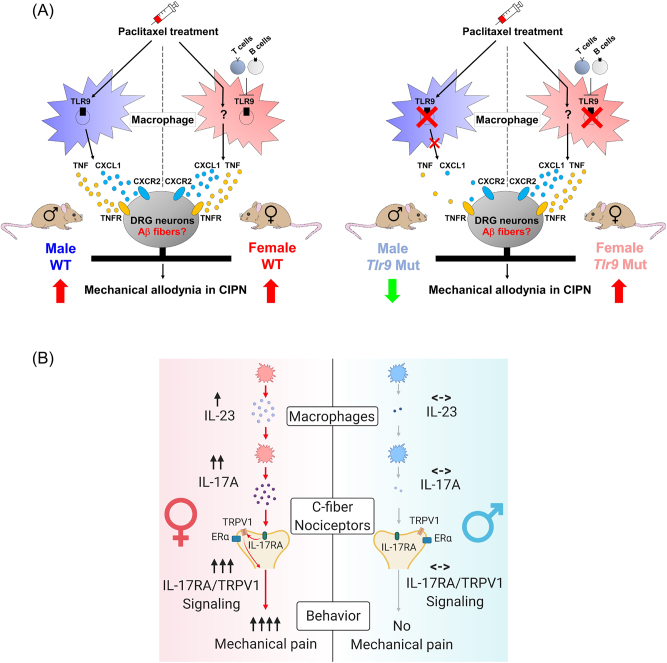
Figure 6:
Distinct macrophage signaling in pain in male and female mice. (A) Macrophage toll-like receptor 9 (TLR9) regulates mechanical pain in male mice. Left and right panels: schematic for macrophage TLR9 regulation of mechanical allodynia in chemotherapy-induced peripheral neuropathy (CIPN) in WT and Tlr9 mutant mice. Left, paclitaxel induces the infiltration and activation of macrophages in dorsal root ganglions (DRGs) in mice of both sexes. Paclitaxel also promotes macrophage release of TNF and CXCL1 in DRGs with subsequent binding to receptors TNFR1/R2 and CXCR2 on sensory neurons, thereby leading to hyperexcitability in nociceptive DRG neurons and driving mechanical allodynia in CIPN. Right, blocking TLR9 attenuates the development of paclitaxel (PTX)-elicited mechanical allodynia via downregulation of the PTX-induced macrophage release of TNF and CXCL1 only in male but not female mice. Additionally, female mice deficient in T and B cells may switch to utilizing TLR9 signaling in CIPN. Reproduced from Luo et al. [116] with permission (author’s own rights). (B) Macrophage signaling in female-dependent mechanical pain. Schematic illustration of female-specific modulation of mechanical pain by IL-23/IL-17A/transient receptor potential ion channel subtype V1 (TRPV1) axis. Note that both macrophage and nociceptor signaling contribute to the sex dimorphism. Reproduced from Luo et al. [119] with CCC permission.