Abstract
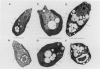
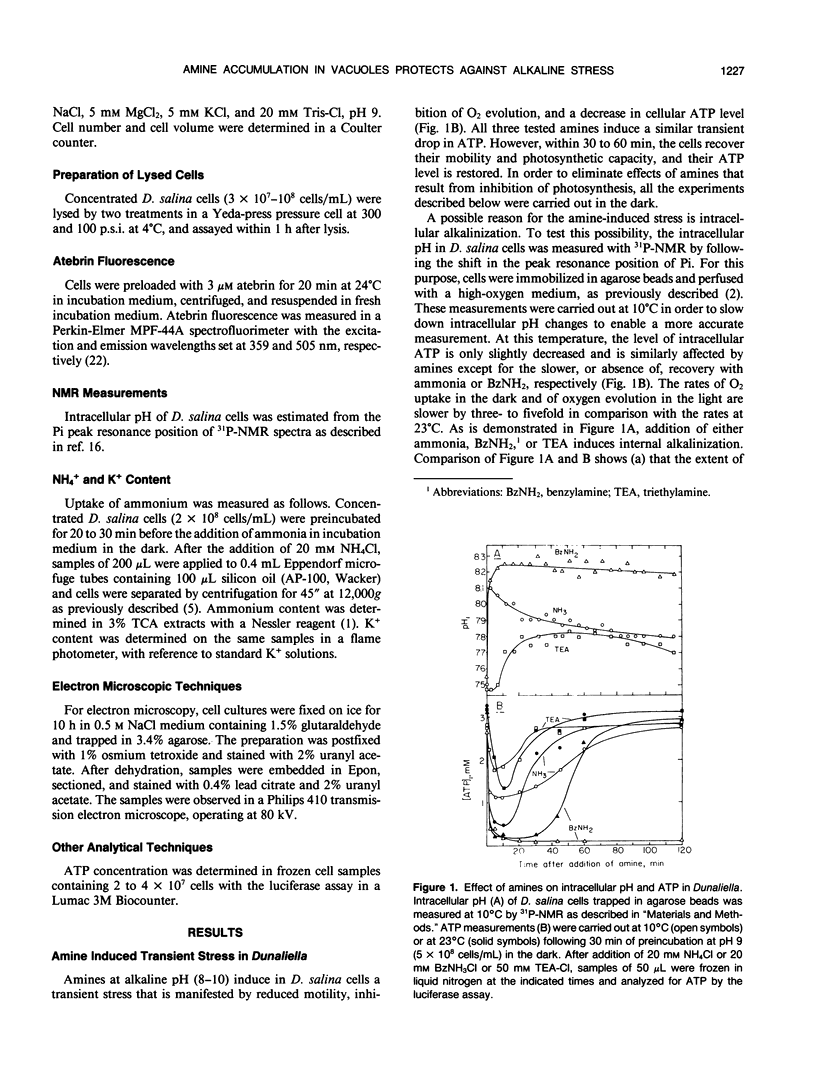
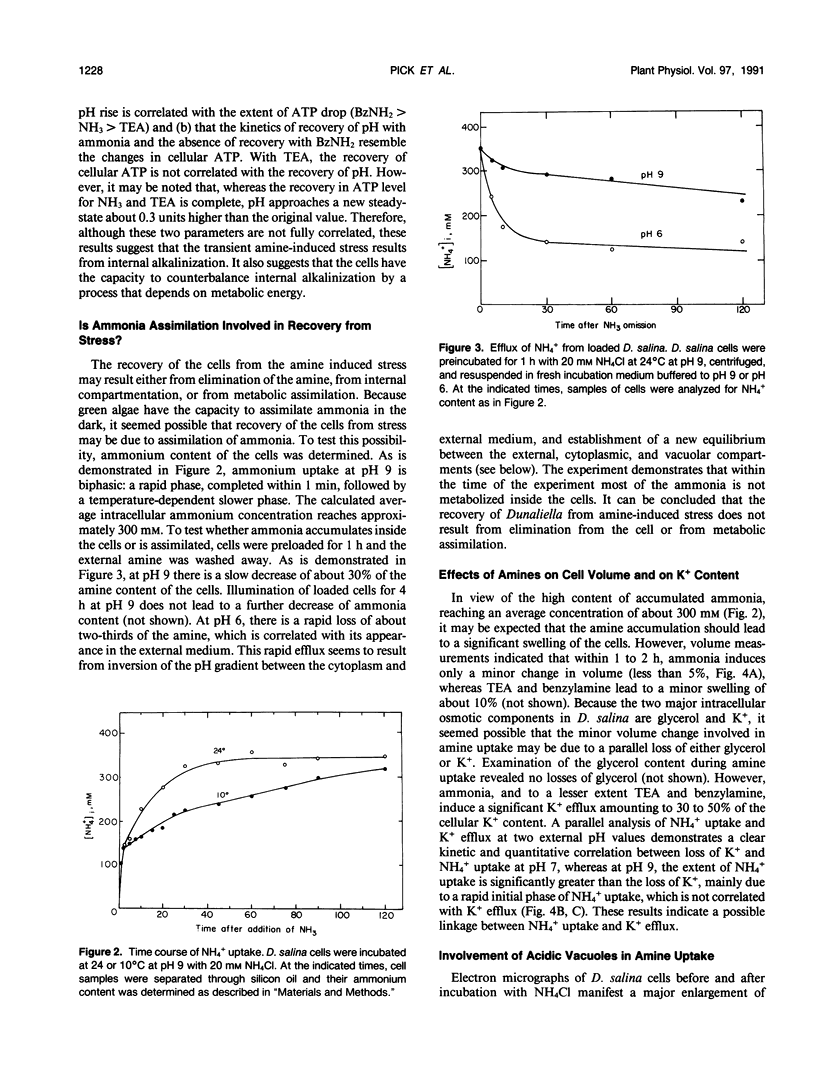
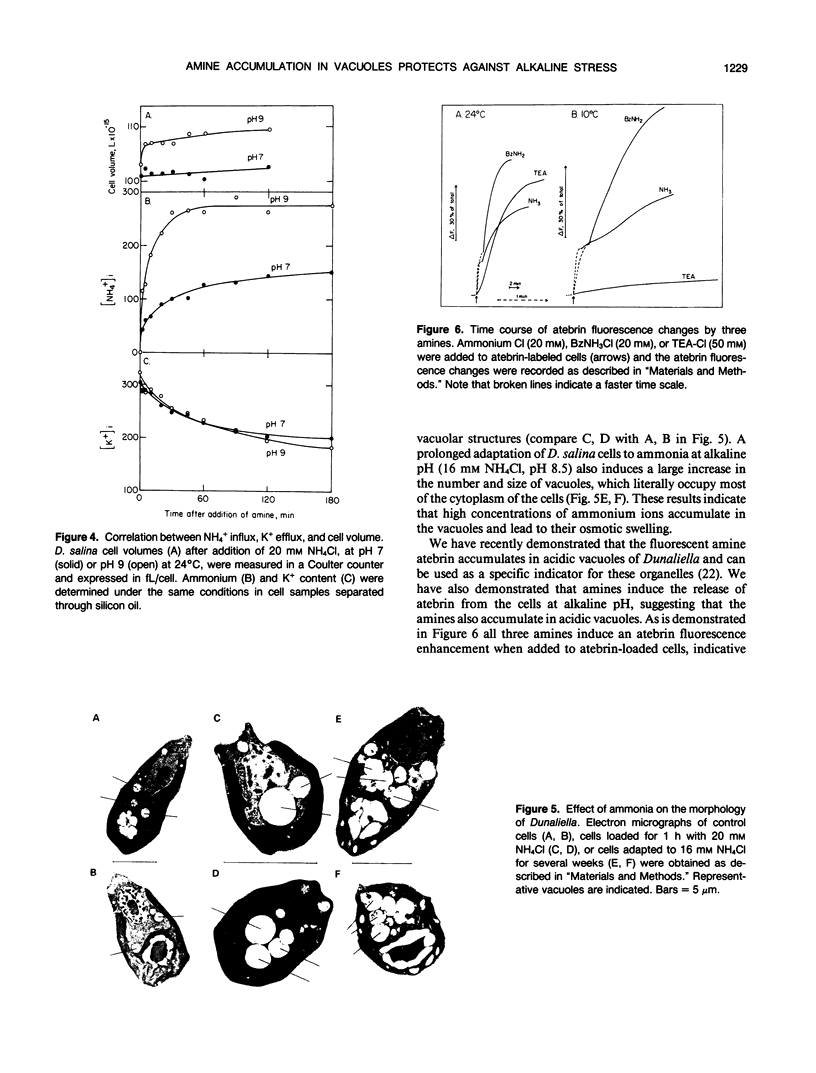
Amines at alkaline pH induce in cells of the halotolerant alga Dunaliella a transient stress that is manifested by a drop in ATP and an increase of cytoplasmic pH. As much as 300 millimolar NH4+ are taken up by the cells at pH 9. The uptake is not associated with gross changes in volume and is accompanied by K+ efflux. Most of the amine is not metabolized, and can be released by external acidification. Recovery of the cells from the amine-induced stress occurs within 30 to 60 minutes and is accompanied by massive swelling of vacuoles and by release of the fluorescent dye atebrin from these vacuoles, suggesting that amines are compartmentalized into acidic vacuoles. The time course of ammonia uptake into Dunaliella cells is biphasic—a rapid influx, associated with cytoplasmic alkalinization, followed by a temperature-dependent slow uptake phase, which is correlated with recovery of cellular ATP and cytoplasmic pH. The dependence of amine uptake on external pH indicates that it diffuses into the cells in the free amine form. Studies with lysed cell preparations, in which vacuoles become exposed but retain their capacity to accumulate amines, indicate that the permeability of the vacuolar membrane to amines is much higher than that of the plasma membrane. The results can be retionalized by assuming that the initial amine accumulation, which leads to rapid vacuolar alkalinization, activates metabolic reactions that further increase the capacity of the vacuoles to sequester most of the amine from the cytoplasm. The results indicate that acidic vacuoles in Dunaliella serve as a high-capacity buffering system for amines, and as a safeguard against cytoplasmic alkalinization and uncoupling of photosynthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bental M., Pick U., Avron M., Degani H. Metabolic studies with NMR spectroscopy of the alga Dunaliella salina trapped within agarose beads. Eur J Biochem. 1990 Feb 22;188(1):111–116. doi: 10.1111/j.1432-1033.1990.tb15377.x. [DOI] [PubMed] [Google Scholar]
- Bertl A., Felle H., Bentrup F. W. Amine Transport in Riccia fluitans: Cytoplasmic and Vacuolar pH Recorded by a pH-Sensitive Microelectrode. Plant Physiol. 1984 Sep;76(1):75–78. doi: 10.1104/pp.76.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D. The transport of NH3 and NH4+ across biological membranes. Biochim Biophys Acta. 1981 Nov 9;639(1):41–52. doi: 10.1016/0304-4173(81)90004-5. [DOI] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A., Leguay J. J., Guern J. Measurement of intracellular pH and aspects of its control in higher plant cells cultivated in liquid medium. Respir Physiol. 1978 Apr;33(1):75–89. doi: 10.1016/0034-5687(78)90086-5. [DOI] [PubMed] [Google Scholar]
- Maeda M., Thompson G. A., Jr On the mechanism of rapid plasma membrane and chloroplast envelope expansion in Dunaliella salina exposed to hypoosmotic shock. J Cell Biol. 1986 Jan;102(1):289–297. doi: 10.1083/jcb.102.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Ben-Amotz A., Karni L., Seebergts C. J., Avron M. Partial Characterization of K and Ca Uptake Systems in the Halotolerant Alga Dunaliella salina. Plant Physiol. 1986 Jul;81(3):875–881. doi: 10.1104/pp.81.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Bental M., Chitlaru E., Weiss M. Polyphosphate-hydrolysis--a protective mechanism against alkaline stress? FEBS Lett. 1990 Nov 12;274(1-2):15–18. doi: 10.1016/0014-5793(90)81318-i. [DOI] [PubMed] [Google Scholar]
- Pick U., Weiss M. Polyphosphate Hydrolysis within Acidic Vacuoles in Response to Amine-Induced Alkaline Stress in the Halotolerant Alga Dunaliella salina. Plant Physiol. 1991 Nov;97(3):1234–1240. doi: 10.1104/pp.97.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Wemmer D., Ray P. M., Jardetzky O. Regulation of Cytoplasmic and Vacuolar pH in Maize Root Tips under Different Experimental Conditions. Plant Physiol. 1982 Jun;69(6):1344–1347. doi: 10.1104/pp.69.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Correlation between photosynthesis and the transthylakoid proton gradient. Biochim Biophys Acta. 1981 Apr 13;635(2):393–404. doi: 10.1016/0005-2728(81)90037-2. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe G. C., Schuller K. A., Smith R. G., Feil R., Plaxton W. C., Turpin D. H. Relationship between NH(4) Assimilation Rate and in Vivo Phosphoenolpyruvate Carboxylase Activity : Regulation of Anaplerotic Carbon Flow in the Green Alga Selenastrum minutum. Plant Physiol. 1990 Sep;94(1):284–290. doi: 10.1104/pp.94.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]