Figure 1.
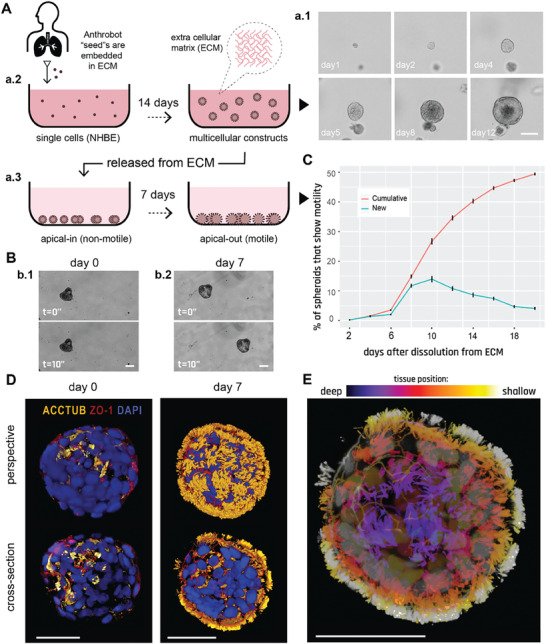
Human bronchial epithelial cells self‐construct into multicellular motile living architectures. A) Workflow for producing Anthrobots. NHBE cells’ apical‐in to apical‐out transition is facilitated by first culturing them in extra cellular matrix (ECM) under appropriate differentiation‐inducing conditions, during which time apical‐in spheroids self‐construct from single cells a.1), and upon the completion of this 14 day period a.2) by releasing mature spheroids from the ECM a.3) and continuing to culture them in low‐adhesive environment. B) Phase contrast images of an apical‐in b.1) and apical‐out b.2) spheroids, captured immediately after dissolution from ECM (day 0) and 7 days after dissolution (day 7), respectively. Day 0 spheroids show no motility, whereas day 7 spheroids show drastically increased motility. C) Percentage of cumulative (total fraction of motile spheroid since day 0) and newly motile spheroids (fraction of motile spheroid that reached motility since the previous time point) in the 3 weeks following dissolution. Out of the 2281 spheroids characterized total, ≈50% consistently showed no signs of motility (despite most having cilia) within this 3‐week period and are referred to as non‐movers. The data shown on this graph only include the motile bots, N = 1127. D) Immunostaining of two separate spheroids from day 0 and day 7 with a‐tubulin (cilia marker), Zonula occludens (ZO)‐1 (tight junction marker), and the nuclear stain 4',6‐diamidino‐2‐phenylindole (DAPI). Amount of multiciliate cells on the spheroid surface show a drastic increase by day 7. E) A day 7 Anthrobot with depth information to show full cilia coverage. Bots in panels D,E were immunostained with α‐tubulin (cilia marker), ZO‐1 (tight junction marker), and DAPI (nuclear stain). Colors represent tissue depth. All scalebars on this figure feature 50 um.
