Abstract
In early 1999, there was an increased incidence of tuberculous lesions in the lymph nodes of slaughtered pigs in the Czech Republic. In part 1 of this study, tuberculous lesions were detected in 140 (62%) tissue samples collected from pigs coming from 15 farms in 15 districts at routine veterinary meat inspections in abattoirs. Mycobacteria were isolated from 37 (16%) tissue samples: 34 Mycobacterium avium subsp. hominissuis isolates and three environmentally derived mycobacteria. In search of infection sources, M. avium subsp. hominissuis was isolated from 38 (79%) samples of peat used as a feed supplement. In part 2 of our study, the head, mesenteric, and inguinal lymph nodes of 117 randomly selected slaughtered pigs from one farm with young piglets fed peat as a supplement were investigated for mycobacterial infection. From 65 (56%) pigs, a total of 76 mycobacterial isolates were identified (56 M. avium subsp. hominissuis isolates, 5 M. avium subsp. avium isolates, 3 M. intracellulare isolates, and 12 environmentally derived mycobacterial isolates). IS1245 restriction fragment length polymorphism (RFLP) types with >20 bands of 45 distinct RFLP types were found in 49 M. avium subsp. hominissuis isolates from pigs (n = 31) and peat (n = 18). Identical RFLP types were found in only four pig isolates. Five randomly selected isolates from pigs and peat were subcultured to six independent clones or colonies. Among the IS1245 RFLP types of 30 clones, identical RFLP types obtained from pigs and peat were identified, which confirmed the hypothesis that peat contaminated with mycobacteria represents a significant source of mycobacterial infection for pigs.
Infections of pigs caused by Mycobacterium avium complex (MAC) organisms result in severe financial losses for farmers in a number of countries. These economic losses primarily result from condemnation of pig meat and head and visceral organs at abattoir inspection points due to tuberculous lesions. In some circumstances, such meat and organs are assessed as conditionally consumable subject to thorough heat treatment (2, 6, 29, 30, 43, 47). Further losses occur from prohibition of the sale and movement of live animals from infected farms (48). The most important member of the MAC is M. avium subsp. avium IS901+, IS1245+, and serotypes 1 to 3 (41), which is virulent for birds. This organism is commonly isolated from wild and domestic birds and small rodents (17, 44, 49). Conversely, MAC isolates of genotypes IS901− and IS1245+ and serotypes 4 to 6, 8 to 11, and 21 are less virulent for birds and are designated M. avium subsp. hominissuis (41); they are most often isolated from various environmental objects (water, soil, dust, invertebrates, and other materials). The other member of the MAC, M. intracellulare of genotypes IS901− and IS1245− and serotypes 7, 12 to 20, and 22 to 28, and other conditionally pathogenic mycobacterial species are nonvirulent for birds and are commonly found in the environment (18, 27, 28, 60).
From the standpoint of contamination by these bacteria, drinking water (3, 25, 38), feed (25, 27, 38), bedding materials (5, 7, 37, 38, 54), soil in pig runs (27, 37, 38), wastewater (27, 37), invertebrates (16-20, 38, 39, 62), and other materials are particular sources of mycobacterial infections for pigs. Environmentally derived mycobacteria can sensitize pigs and other domestic animals, which results in nonspecific reactions to tuberculin skin tests. This immunological interference usually complicates intravital diagnosis (especially skin and serological testing) of tuberculosis in animals (21, 28, 42, 50). Infections with MAC organisms, particularly with M. avium subsp. avium and M. avium subsp. hominissuis, can elicit tuberculous lesions in head, jejunal, and ileocecal lymph nodes. At abattoir meat inspection points, these lesions cannot easily be distinguished from tuberculous alterations and usually complicate the control of bovine tuberculosis in livestock (9, 36, 43, 57).
In the Czech Republic, supplementing the diet of newborn piglets with peat began in 1998 (38, 48). Beyond its good dietary characteristics, the low pH (4.0 to 4.5) and bactericidal effect of peat inactivate coliform bacteria and other bacterial species of the intestinal microflora and subsequently reduce or completely eliminate bacterial toxins in the intestine (22, 28, 35). Addition of peat to the diet of piglets improves feed intake and consequently increases daily weight gain (23, 53). The relatively high fiber content of peat enhances absorption of water in the intestine and mechanically extends the piglets' stomachs. In some herds, because of its soft texture, which prevents limb abrasions in piglets, peat is used as a bedding material (11).
Nevertheless, the low pH of peat provides a favorable environment for mycobacteria. Particularly environmentally derived mycobacteria may be propagated in peat if the temperature is higher than 18°C (28). As environmentally derived mycobacteria are conditionally pathogenic, this may cause tuberculous lesions in pig lymph nodes. Observation of the good prophylactic effect of peat on intestinal infections in piglets led some farmers to provide peat to all neonates. After several months of feeding, however, abattoir inspection revealed tuberculous lesions in head and mesenteric lymph nodes (48).
The study was carried out in two parts. The objective of part 1 was to determine the incidence of mycobacteria in pig organs and in samples of the environment from 15 pig herds in the Czech Republic in which peat was used as a feed supplement. Part 2 investigated the distribution of tuberculous lesions in the head, mesenteric, and inguinal lymph nodes of pigs fed peat coming from one large farm.
MATERIALS AND METHODS
A total of 577 pig organs and 220 samples of farm environment were examined in parts 1 and 2 of this study.
In part 1, a total of 226 tissue samples from 185 pigs were collected from abattoirs during routine meat inspections. The animals originated from 15 swine farms in which peat was added to the diet of piglets during the first 2 to 4 weeks of life. In an attempt to identify the sources of mycobacterial infections of pigs, 87 environmental samples of the farms' premises were examined as follows: 39 samples of water, feed, bedding materials, scrapings from the barn, and dust (2 to 3 samples from each farm) and 48 samples of peat with which the piglets' diets were supplemented (2 to 4 samples from each farm).
Part 2 was concerned with an investigation of the presence of tuberculous lesions in the head, mesenteric, and inguinal lymph nodes of 117 randomly selected slaughtered pigs with a mean weight of 115 kg that came from one specific swine farm. A total of 133 samples of peat with which the piglets' feed was supplemented were tested.
Detection of mycobacteria. (i) Sample collection.
In part 1, at least one sample of the head, mesenteric, and inguinal lymph nodes was collected from each animal; in part 2, three lymph nodes (head, mesenteric, and inguinal) were collected from each animal. The lymph nodes were individually collected in sterile polyethylene bags with sterile scissors and forceps. The samples were marked with the names of the lymph nodes and the identification of the slaughtered animal.
(ii) Sample storage.
After collection, organ samples were transported to the laboratory in insulated boxes with ice packs at 4°C. Tissues for histological examinations were immersed in 10% formalin. Organ samples were frozen at −18°C and kept for up to 3 weeks until they were processed, whereas the environmental samples were kept at 6°C in a dark room for up to 2 weeks.
(iii) Histopathology.
In part 2, 225 samples of the lymph nodes were examined by histopathology. The tissue samples were formalin fixed, embedded in paraffin blocks, and stained by the Ziehl-Neelsen technique for the presence of acid-fast rods (AFR). Histological samples were observed by light microscopy using ×1,000 magnification under oil immersion (Olympus B17 microscope).
(iv) Gross examination, microscopy, and culture examinations.
Tissue samples were rapidly thawed at 37°C and examined for the presence, number, and size of tuberculous lesions. Slides prepared from tissue impressions were stained by the Ziehl-Neelsen technique and examined by light microscopy for the presence of AFR. At least 200 fields of view were examined for each sample. Approximately 1 g of tissue or environmental sample was homogenized with a laboratory blender stomacher (Kleinfeld Labortechnik, GmbH Gehrden, Germany), and the suspension was decontaminated in 1 N HCl for 15 min as previously described (18). The suspension was subsequently neutralized with 2 N NaOH until the color changed to light purple. Phenolphthalein (2%) was used as an indicator. Forty microliters of the suspension was inoculated with sterile disposables tips and dispensed onto two slants each of two solid media and one liquid medium (egg-based media according to the method of Stonebrink; Herrold's egg yolk medium and liquid serum medium according to the method of Sula). The liquid medium contained bovine serum, enzymatic casein hydrolysate, glycerin, l-alanine, phosphate salts, magnesium sulfate, citric salts, and malachite green (31). Incubations were performed simultaneously at two temperatures: one set of the media at 24°C, and the remaining set at 37°C. The cultures were checked after the first week and every 2 weeks until the end of incubation.
(v) Identification of isolates.
All the AFR-positive isolates were examined by PCR for the detection of the dnaJ gene specific for the genus Mycobacterium using the primers 5′-GGG TGA CGC GAC ATG GCC CA-3′ and 5′-CGG GTT TCG TCG TAC TCC TT-3′ (45). For differentiation of the species M. intracellulare and M. avium of the MAC, detection of 850- and 180-bp fragments of 16S rRNA was used (61). For differentiation of subspecies of M. avium, IS901 detection by the primers 5′-GCA ACG GTT GTT GCT TGA AA-3′ and 5′-TGA TAC GGC CGG AAT CGC GT-3′ (32, 33) and IS1245 detection by the primers 5′-GCC GCC GAA ACG ATC TAC-3′ and 5′-AGG TGG CGT CGA GGA AGA-3′ were used (26). All MAC isolates were serotyped according to the system described previously (63) and later modified (55). Mycobacterial isolates that were not classified as MAC were assessed by biochemical methods (60).
(vi) Virulence assessment of M. avium subsp. avium isolates.
For all M. avium subsp. avium isolates, virulence to birds (the ability to produce tuberculous lesions in parenchymatous organs) was tested by intramuscular administration of the pathogen to chickens (49).
(vii) RFLP method.
Five M. avium subsp. avium isolates from pig lymph nodes and one isolate from peat were examined by the restriction fragment length polymorphism (RFLP) method with IS901 and IS1245 hybridization probes described previously (13, 14). DNA was digested with restriction endonucleases (RE) PvuII and PstI. For further RFLP analysis with an IS1245 probe, 49 M. avium subsp. hominissuis isolates were randomly selected. Thirty-one and 18 isolates originated from the preceding 90 and 117 isolates from pigs and peat, respectively. Approximately 5 μg of purified mycobacterial DNA was digested with RE PvuII. DNA fragments were separated by electrophoresis in an agarose gel, exposed to UV light in a transluminator, and transferred from the gel to a nylon membrane by vacuum blotting. The DNA was fixed and hybridized with a labeled probe (IS901 or IS1245) according to a method described previously (13, 14). For the homogeneity-heterogeneity investigation of M. avium subsp. hominissuis isolates after IS1245 RFLP analysis, subculture to six respective clones was performed in two isolates from pigs and three isolates from distinct peat samples (13) each time, hence, in a total of 30 independent clones.
(viii) Designation of RFLP types.
IS901 RFLP profiles were analyzed according to the system described previously (14). IS1245 RFLP profiles were assessed according to the number of bands of either “bird” types (three bands in a conserved pattern) or “nonbird” RFLP types (more than three bands), as reported previously (12, 14, 52, 59).
Statistical evaluation.
The χ2 test was applied for statistical evaluation (40).
RESULTS
Part 1. (i) Tissue samples.
Tuberculous lesions of various sizes were detected in 140 (62%) of 226 tissue samples examined (Table 1). Tuberculous lesions were most frequently detected in head (77%) and mesenteric (73%) lymph nodes. The sizes of tuberculous lesions varied from organ to organ (range, 1 to 10 mm in diameter) (Table 1). In 53% of head lymph nodes, lesions measured up to 1 mm in diameter, and in 23%, lesions up to 5 mm in diameter were observed. However, in mesenteric lymph nodes, the sizes of lesions varied. Nevertheless, large tuberculous lesions (>6 mm in diameter) were more frequently observed in mesenteric lymph nodes (P = 0.05) than in head lymph nodes (Table 1). In one of two lung lymph nodes, the lesion size ranged up to 6 to 10 mm, and 1- to 5-mm-diameter lesions were observed in the liver tissue. No tuberculous lesions were detected in inguinal lymph nodes and muscular tissue samples.
TABLE 1.
Examination for the presence of tuberculous lesions in tissue samples from slaughtered pigs (part 1)
| Origin of tissue samples | Gross examination of tissue samples
|
No. of AFR-positive samples
|
No. of positive samples
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of tissues
|
No. of lesions of diam (mm):
|
Culture
|
Isolates
|
||||||||||
| Examined | Positive | % | ≤1 | 1-5 | 6-10 | ≥10 | PA+b | PA−c | No. | % | MAHd | EMe | |
| Head lnna | 79 | 61 | 77 | 32 | 14 | 8 | 7 | 5 | 1 | 20 | 25 | 19 | 1 |
| Mesenteric lnn | 104 | 76 | 73 | 25 | 18 | 27 | 6 | 3 | 3 | 16 | 15 | 14 | 2 |
| Inguinal lnn | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 4 | 1 | 0 |
| Lung lnn | 2 | 1 | 50 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver | 2 | 2 | 100 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscle tissue | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 226 | 140 | 62 | 57 | 34 | 36 | 13 | 8 | 8 | 37 | 34 | 3 | |
lnn, lymph nodes.
Positive pathologic-anatomical examination for the presence of tuberculous lesions.
Negative pathologic-anatomical examination for the presence of tuberculous lesions.
Isolates of M. avium subsp. hominissuis (genotypes dnaJ+ [180 bp of 16S rRNA], IS901−, and IS1245+ and serotypes 8 and 9).
EM, environmentally derived mycobacteria: one isolate of M. scrofulaceum (head lnn) and two isolates of M. chelonae (mesenteric lnn).
Microscopic examination revealed AFR in 16 (7%) samples, 50% in lymph nodes with tuberculous lesions and 50% in nontuberculous lymph nodes. Culture revealed mycobacteria in only three (19%) of the AFR-positive samples (Table 1).
Mycobacteria were isolated from 37 (16%) of the 226 samples; 34 isolates ranked among M. avium subsp. hominissuis isolates (genotypes dnaJ+ [180 bp of 16S rRNA], IS901−, and IS1245+; serotypes 8 and 9), and the remaining three isolates were classified as environmentally derived mycobacteria (Table 1 and Fig. 1). Thirty-one (84%) mycobacterial isolates were isolated from lymph nodes with tuberculous lesions, and six (16%) were from nontuberculous tissue samples.
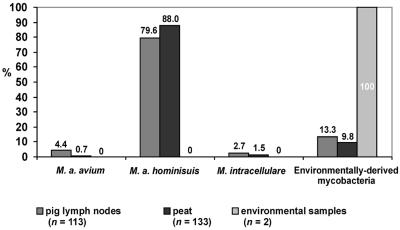
FIG. 1.
Comparison of distributions of 248 mycobacterial isolates from 577 pig lymph nodes, 181 peat samples, and 39 environmental samples (parts 1 and 2).
(ii) Environmental samples.
Out of 39 environmental samples, mycobacteria were isolated from two (5%) samples, whereas 38 (79%) mycobacterial isolates originated from 48 peat samples: 32 isolates belonged to M. avium subsp. hominissuis (genotypes dnaJ+, IS901− [180 bp of 16S rRNA], and IS1245+; serotypes 4, 8, and 9), and six isolates were classified as environmentally derived mycobacteria (Fig. 1).
Part 2. (i) Tissue samples.
Gross tuberculous lesions were found in 81 (69%) of 117 animals and in 140 (40%) of 351 lymph nodes. Histological findings revealed AFR in 38 (17%) of 225 samples examined. In the remaining 187 non-AFR samples, infiltration of monocytes and multinuclear granulocytes with sparse Langerhans cells was observed in 89 (47%) samples. A significantly higher (P = 0.01) proportion of tuberculous lesions were found in head and mesenteric lymph nodes than in inguinal lymph nodes (Table 2).
TABLE 2.
Distribution of mycobacterial infection in 351 tissue samples collected from 117 pigs (part 2)
| Groupa | No. of animals (%) | No. of examined lymph nodesf | No. of positive samples with
|
|||||
|---|---|---|---|---|---|---|---|---|
| Presence of tubercu- lous lesions
|
Acid-fast rod de- tection
|
Isolation of myco- bacteria
|
||||||
| No. | % | No. | % | No. | % | |||
| Animals | ||||||||
| 1b | 36 (31) | 108 | 0 | 0 | 4 | 4 | 12 | 11 |
| 2c | 25 (21) | 75 | 25 | 33 | 0 | 0 | 19 | 25 |
| 3d | 53 (45) | 159 | 106 | 67 | 5 | 3 | 40 | 25 |
| 4e | 3 (3) | 9 | 9 | 100 | 0 | 0 | 5 | 56 |
| Total | 117 (100) | 351 | 140 | 40 | 9 | 3 | 76 | 22 |
| Lymphnodes | ||||||||
| Head | 68 | 58 | 4 | 3 | 47 | 40 | ||
| Mesenteric | 66 | 56 | 2 | 2 | 25 | 21 | ||
| Inguinal | 6 | 5 | 3 | 3 | 4 | 3 | ||
Groups according to the results of pathologic-anatomical examination.
Animals without tuberculous lesions.
Animals with tuberculous lesions in one lymph node only (12 animals had lesions in head lymph nodes, 11 animals had lesions in mesenteric lymph nodes, and 2 animals had lesions in inguinal lymph nodes).
Animals with tuberculous lesions in two lymph nodes (52 animals had lesions simultaneously in head and mesenteric lymph nodes, and only 1 animal had lesions simultaneously in mesenteric and inguinal lymph nodes).
Animals with tuberculous lesions in three lymph nodes.
In every animal, head, mesenteric, and inguinal lymph nodes were tested.
The distributions of tuberculous lesions in the head, mesenteric, and inguinal lymph nodes of 140 slaughtered pigs are depicted in Table 2. In group 1 (31% of the animals), tuberculous lesions were not detected. In group 2 (21% of the animals), tuberculous lesions were detected in only one of the lymph node groups. In the inguinal lymph nodes of two pigs in which pathological lesions were observed, mycobacteria were detected. In group 3 (45% of the animals), tuberculous lesions were found in two lymph node groups: in the head and mesenteric lymph nodes of 52 animals and in the head and inguinal lymph nodes of one animal. In group 4 (3% of the animals), tuberculous lesions were found in all lymph nodes. Tuberculous lesions were not concurrently detected in mesenteric and inguinal lymph nodes in any of the animals.
AFR were detected in only 9 (3%) of 351 samples of lymph nodes that originated from eight animals (7%). In one pig, mycobacteria were microscopically detected in the mesenteric and inguinal lymph nodes (Table 2).
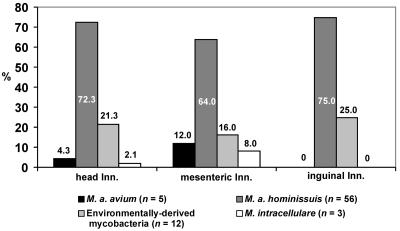
Mycobacteria were isolated from 76 (22%) samples out of 351 examined; 58 (76%) isolates were from lymph nodes with tuberculous lesions, and 18 (24%) isolates were from lymph nodes without lesions. Five M. avium subsp. avium (genotypes dnaJ+ [180 bp of 16S rRNA], IS901+, and IS1245+) isolates were of serotype 2, and 56 M. avium subsp. hominissuis (genotypes dnaJ+ [180 bp of 16S rRNA], IS901−, and IS1245+) isolates were of serotypes 6 (n = 1), 8 (n = 47), and 9 (n = 8). Three M. intracellulare isolates were of genotypes dnaJ+ (850 bp of 16S rRNA), IS901−, and IS1245−; serotyping was not possible because of autoagglutination. The remaining 12 isolates were identified by biochemical tests as environmentally derived mycobacteria (Fig. 1 and 2).
FIG. 2.
Distribution of 76 mycobacterial isolates from lymph nodes of 117 pigs fed with peat as a feed supplement (part 2).
Comparison of gross, microscopic, and culture examinations demonstrated that a significantly high (P = 0.01) proportion of the head lymph nodes were infected, and overall, infection of 95 (81%) pigs was observed. Of the 36 animals (group 1) in which no gross tuberculous lesions were observed, mycobacteria were detected microscopically and/or by culture in 14 (39%) animals (Table 2).
Analysis of mycobacterial species isolated from lymph nodes revealed that M. avium subsp. avium isolates were detected in head and mesenteric lymph nodes only. No statistically significant differences were found between the distributions of M. avium subsp. hominissuis isolates. M. intracellulare isolates were detected in head and mesenteric lymph nodes (Fig. 1 and 2).
(ii) Peat samples.
Culture examinations detected mycobacterial isolates in 95 (71%) samples. However, one M. avium subsp. avium (genotype dnaJ+ [180 bp of 16S rRNA], IS901+, IS1245+; serotyping was not done due to autoagglutination) isolate was detected (Fig. 1).
(iii) Virulence testing in pullets.
All five M. avium subsp. avium isolates from pig lymph nodes caused tuberculosis of parenchymatous organs in infected pullets 6 to 8 weeks after intramuscular administration. An M. avium subsp. avium isolate obtained from a peat sample was not virulent for pullets, and the tuberculous lesions were found in the site of inoculation (breast muscles) only.
(iv) IS901 RFLP profiles.
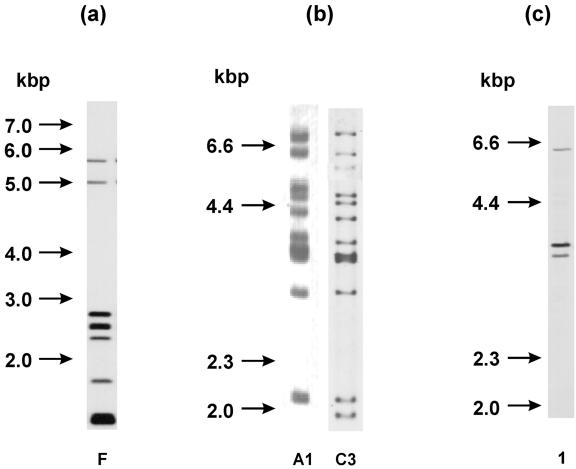
By the IS901 RFLP method, one F-A1 RFLP type was obtained from five M. avium subsp. avium isolates originating from pig lymph nodes, and another F-C3 RFLP type was obtained from an M. avium subsp. avium isolate from peat (Fig. 3a and b). In all six M. avium subsp. avium isolates, the same bird IS1245 RFLP type was detected (Fig. 3c).
FIG. 3.
(a) IS901 RFLP types of M. avium subsp. avium isolates after using RE PvuII. RFLP type F was detected in five pig isolates and one peat isolate. RFLP type designation was performed according to the method of Dvorska et al. (14). (b) IS901 RFLP types of M. avium subsp. avium isolates after using RE PstI. Shown are RFLP type A1 of one of five identical pig isolates and RFLP type C3 of one peat isolate. RFLP type designation was performed according to the method of Dvorska et al. (14). (c) IS1245 RFLP types of M. avium subsp. avium isolates. Bird RFLP types were detected in five isolates from pigs and one isolate from peat (line 1). RFLP type designation was performed according to the method of Ritacco et al. (52).
IS1245 RFLP profiles of isolates from parts 1 and 2.
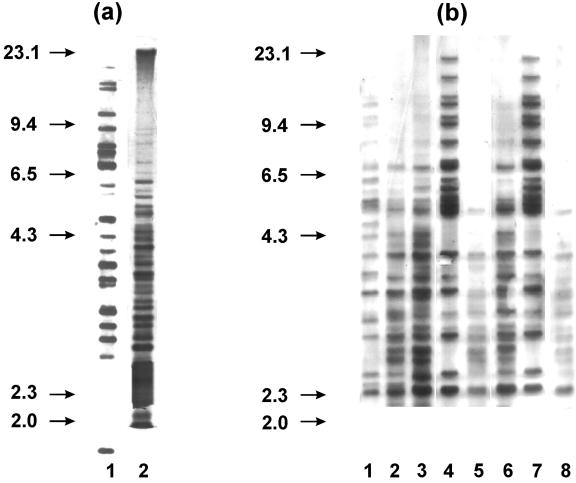
Significant heterogeneity was detected among 49 randomly selected M. avium subsp. hominissuis isolates by IS1245 RFLP analysis. Forty-five different RFLP types with >20 bands in a genome were observed. Identical RFLP types were detected only in two distinct M. avium subsp. hominissuis isolates from pigs and in two M. avium subsp. hominissuis isolates from various peat samples (Fig. 4a).
FIG. 4.
(a) IS1245 RFLP types of M. avium subsp. hominissuis isolates. Line 1, identical RFLP types of two isolates from pigs; line 2, identical RFLP types of two isolates from peat. RFLP type designation was performed according to the method of Ritacco et al. (52). (b) IS1245 RFLP types of pig and peat M. avium subsp. hominissuis isolates and their clones. Line 1, RFLP type of one pig isolate; lines 2 to 4, RFLP types of its clones; line 5, RFLP type of one peat isolate; lines 6 to 8, RFLP types of its clones. RFLP type designation was performed according to the method of Ritacco et al. (52).
Among RFLP types of 30 clones obtained from five field isolates from pigs (n = 2) and peat (n = 3) originating from the same locality, identical RFLP types in one clone from peat and one clone obtained by subculture of a pig isolate were detected in two cases (Fig. 4b). In all five cases, ≥2 different RFLP types were detected in six clones originating from one isolate.
Biochemical identification of environmentally derived mycobacteria (parts 1 and 2).
In part 1, M. fortuitum and M. gordonae were isolated from environmental samples. Mycobacterial isolates from the peat samples taken in parts 1 and 2 were identified as M. fortuitum (three times), M. gordonae (three times), M. chelonae (two times), M. terrae (one time), M. xenopi (one time), M. flavescens (one time), M. phlei (one time), and M. scrofulaceum (one time).
In parts 1 and 2, M. chelonae (six times), M. fortuitum (three times), M. scrofulaceum (three times), M. terrae (two times), and M. smegmatis (one time) were isolated from pig samples (Table 1 and Fig. 1).
DISCUSSION
Mycobacteria were isolated more frequently from samples of peat used as a feed supplement for pigs than from other samples of the external environment (Fig. 1). Detection of the same mycobacterial species in different lymph nodes of slaughtered pigs that received a peat-supplemented diet before weaning supports our hypothesis implicating peat as a source of mycobacterial infection for piglets. M. avium subsp. hominissuis was predominantly detected in peat and pig organs. Various species of environmentally derived mycobacteria and M. intracellulare represented ∼10% of these isolates. In the peat originating from natural sources (sphagnum vegetation), M. sphagni and other nonpathogenic and conditionally pathogenic mycobacteria are frequently available. However, isolation of M. fortuitum, M. terrae, M. chelonae, M. gordonae, M. xenopi, M. phlei, M. marinum, M. flavescens, M. farcinogenes, and M. scrofulaceum is not uncommon (28). Except for M. sphagni, most of these organisms were demonstrated in peat samples in our study (4).
As commercially prepared peat is mined from the lower parts of peat bogs, it is supposed to be sterile and free from mycobacteria. When transportation of peat and other manipulations are performed for several months, contamination with mycobacteria from environmental sources, such as dust, water, and feces of animals, is likely (3, 27, 28, 38).
The M. avium subsp. avium isolate that was detected in peat showed a different IS901 RFLP type than the M. avium subsp. avium isolates detected in the tissues of pigs. This discrepancy could be attributed to other sources of infection that might have originated from wild birds harboring M. avium subsp. avium. Previous studies have reported wild birds as a possible source of avian tuberculosis (10, 15, 27, 49).
M. avium subsp. avium isolated from peat was not virulent for pullets; however, the M. avium subsp. avium isolates from the tissues of the pigs were virulent. In our previous study, we found loss of virulence for birds by a culture collection of strains of M. avium subsp. avium obtained from atypical hosts (e.g., humans) or kept in collections for a long time (14, 49). Therefore, our isolation of nonvirulent M. avium subsp. avium from the peat sample could be caused by an “old contamination” of the sphagnum vegetation by wild birds.
Identification of MAC isolates by PCR and serotyping mainly showed the presence of M. avium subsp. hominissuis isolates of serotypes 8 and 9. As described previously, those mycobacteria are the most common causes of tuberculous lesions in pigs (1, 2, 7, 8, 15, 29, 34, 41, 44, 46-49, 51, 52, 62). During the years 1990 to 1999 in the Czech Republic, 7,246 mycobacterial isolates were obtained from tissue samples of slaughtered pigs; 0.1, 94.9, and 5.1% of them ranked among M. bovis, MAC, and environmentally derived mycobacteria (like M. fortuitum, M. terrae, M. chelonae, and M. phlei), respectively (48).
Microscopy examination showed low sensitivity because AFR organisms were detected in only 4% of the lymph nodes. Culture detected mycobacteria in only 19% of microscopically positive samples. This could be attributed to the cellular immune response of the host organism, which may inactivate mycobacteria and contain infection within the lymph nodes. Consequently, these impaired mycobacterial cells could not be recovered by conventional culture methods. Therefore, samples from microscopically positive tissue impression smears may not grow on primary cultures. Culture recovery of these damaged or inactivated mycobacteria could be improved by the use of enriched liquid media, such as MGIT (Becton Dickinson).
Our detection of different sizes of tuberculous lesions in various lymph nodes of slaughtered pigs is consistent with previous publications (58). Tuberculous lesions in head lymph nodes tended to be <5 mm in diameter, while larger tuberculous lesions ranging from 5 to 10 mm in diameter were found in mesenteric lymph nodes. In the advanced process of pathogenesis, progressive calcification and devitalization of mycobacteria within the lesions occur (58); thus, isolation and subsequent identification of mycobacteria by culture examination is difficult. The causes of granuloma formation may also be other species of bacteria, e.g., Rhodococcus equi (15, 24, 48, 56).
During investigation of tuberculous-lesion localizations in the lymph nodes, lesions were mostly detected in head and mesenteric lymph nodes in both parts 1 and 2 of our study. During repeated intake of peat, it is likely that large numbers of mycobacteria penetrate through the mucosa of the mouth and the gastrointestinal tract and pass via lymphatic drainage to the head and mesenteric lymph nodes. No statistically significant differences were found between the findings in head and mesenteric lymph nodes (Tables 1 and 2). The majority of infections are contained in the local lymph nodes of the gastrointestinal tract and do not spread to other lymph nodes or organs.
From the standpoint of food safety, the finding of tuberculous lesions only in the inguinal lymph nodes of six pigs and the detection of tuberculous lesions in the liver and lung lymph nodes (Table 1) should be considered potentially serious. Findings of large tuberculous lesions in the liver and lung lymph nodes are uncommon, and the isolation of mycobacteria from those organs is usually difficult (2, 29). Balian et al. published similar results showing tuberculous lesion detection in liver samples and muscles of pigs (2). The absence of tuberculous lesions was registered in only 22 (19%) animals, in which mycobacteria were not detected either by culture or by microscopic examination. The results obtained in our study show a strong infective pressure of mycobacterial agents on piglets.
In pigs in which the mycobacterial distribution in lymph nodes was investigated, similar percentages of tuberculous lesions in head and mesenteric lymph nodes were registered. Findings of tuberculous lesions localized in only one group of lymph nodes were less frequent. As there was no other information about the influence of peat on the distribution of mycobacteria in pig lymph nodes, we could compare our results only with the influence of feedstuffs containing mycobacteria on the distribution of infections in pigs (7). In this case, tuberculous lesions were detected in mesenteric and submandibular lymph nodes and in both lymph nodes in 78, 6, and 16% of the pigs, respectively. Most MAC isolates described by Dalchow (7) were of serotype 8 (according to the present taxonomy of M. avium subsp. hominissuis), which is the same serotype as in some isolates detected in our study (Fig. 2).
Most of the mycobacterial species isolated from head and mesenteric lymph nodes were similar. Implication of peat as a source of mycobacterial infection for pigs is also supported by the results (Fig. 1) in parts 1 and 2 of our study, in which among IS1245 RFLP types of 30 clones, identical RFLP types of M. avium subsp. hominissuis were obtained from animal tissues and peat.
Recent studies have reported an increased rate of MAC infections, particularly M. avium subsp. hominissuis, in immunocompromised patients. Molecular biological studies proved that pigs were significant sources of avian tuberculosis infections for humans (49). Based on the IS1245 RFLP method, Komijn et al. (29) detected a minimum of 75% similarity with RFLP of M. avium subsp. hominissuis in 61 and 59% of human and pig isolates, respectively (29).
Acknowledgments
We thank Ludvik Maurenc from the Veterinary Hygiene Centre of the abattoir in Pisek (Czech Republic) for collecting some of the pig lymph node samples. The plasmid pMA12 with the cloned IS1245-specific fragment was kindly provided by Pieter Overduin of the RIVM Bilthoven (The Netherlands). Thanks are due to Colin MacIntosh of AgResearch Ltd. (Invermay Agricultural Centre, Mosgiel, New Zealand) for comments and critical reading of the manuscript.
This work was supported by grants no. QC0195, 1B 53009, and MZE 0002716201 from the Grant Agency of the Ministry of Agriculture of the Czech Republic.
REFERENCES
- 1.Alfredsen, S., and E. Skjerve. 1993. An abattoir-based case-control study of risk-factors for mycobacteriosis in Norwegian swine. Prev. Vet. Med. 15:253-259. [Google Scholar]
- 2.Balian, S. C., P. Ribeiro, S. A. Vasconcellos, S. R. Pinheiro, J. S. Ferreira Neto, J. L. Guerra, J. G. Xavier, Z. M. Morais, and M. A. Telles. 1997. Tuberculosis lymphadenitis in slaughtered swine from the State of Sao Paulo, Brazil: microscopic histopathology and demonstration of mycobacteria. Rev. Saude Publica 31:391-397. [DOI] [PubMed] [Google Scholar]
- 3.Beerwerth, W. 1973. Mykobakterien in Viehtränken und Oberflächengewässern. Dtsch. Tierärztl. Wochenschr. 80:398-401. [PubMed] [Google Scholar]
- 4.Bercovier, H., and V. Vincent. 2001. Mycobacterial infections in domestic and wild animals due to Mycobacterium marinum, M. fortuitum, M. chelonae, M. porcinum, M. farcinogenes, M. smegmatis, M. scrofulaceum, M. xenopi, M. kansasii, M. simiae and M. genavense. Rev. Sci. Technol. 20:265-290. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, O. H. 1971. Observations on outbreaks of Battey type mycobacteriosis in pigs raised on deep litter. Austr. Vet. J. 47:424-427. [DOI] [PubMed] [Google Scholar]
- 6.Cvetnic, Z., H. Kovacic, and M. Ocepek. 1998. Mycobacteria in the environment and in the feed of swine in Croatia. Sien. Tierarztl. Monatssch. 85:18-21. (In German.) [Google Scholar]
- 7.Dalchow, W. 1988. Mycobacteriosis in pigs fed cereal waters. Tierärztl. Umsch. 43:62-74. (In German.) [Google Scholar]
- 8.Dalchow, W., and J. Nassal. 1979. Mycobacterial disease in swine caused by use of sawdust for litter. Tierärztl. Umsch. 34:253-261. (In German.) [Google Scholar]
- 9.Dey, B. P., and G. L. Parham. 1993. Incidence and economics of tuberculosis in swine slaughtered from 1976 to 1988—food animal economics. J. Am. Vet. Med. Assoc. 203:516-519. [PubMed] [Google Scholar]
- 10.di Guardo, G., G. de Angelis, and P. L. Longo. 1991. Atypical M. avium induced tubercular lesions in pigs. Vet. Rec. 129:476. [DOI] [PubMed] [Google Scholar]
- 11.Dürrling, H., F. Ludewig, J. Uhlemann, and R. Gericke. 1998. Peat as a source of Mycobacterium avium infection for pigs. Tierärztl. Umsch. 53:259-261. (In German.) [Google Scholar]
- 12.Dvorska, L., M. Bartos, G. Martin, W. Erler, and I. Pavlik. 2001. Strategies for differentiation, identification and typing of medically important species of mycobacteria by molecular methods. Veterinarni Medicina 46:309-328. [Google Scholar]
- 13.Dvorska, L., M. Bartos, O. Ostadal, J. Kaustova, L. Matlova, and I. Pavlik. 2002. IS1311 and IS1245 restriction fragment length polymorphism analyses, serotypes, and drug susceptibilities of Mycobacterium avium complex isolates obtained from a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 40:3712-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorska, L., T. Bull, M. Bartos, L. Matlova, P. Svastova, R. T. Weston, J. Kintr, I. Parmova, D. Van Soolingen, and I. Pavlik. 2003. A standardised restriction fragment length polymorphism (RFLP) method for typing Mycobacterium avium isolates links IS901 with virulence for birds. J. Microbiol. Methods 55:11-27. [DOI] [PubMed] [Google Scholar]
- 15.Dvorska, L., I. Parmova, M. Lavickova, J. Bartl, V. Vrbas, and I. Pavlik. 1999. Isolation of Rhodococcus equi and atypical mycobacteria from lymph nodes of pigs and cattle in herds with the occurrence of tuberculoid gross changes in the Czech Republic over the period of 1996-1998. Veterinarni Medicina 44:321-330. [Google Scholar]
- 16.Engel, H. W. B., D. G. Groothuis, W. Wouda, C. D. W. König, and L. H. H. M. Lendfers. 1977. “Pig-compost” as a source of Mycobacterium avium infection in swine. Zentbl. Vet. Med. B 25:373-382. (In German.) [DOI] [PubMed] [Google Scholar]
- 17.Fischer, O., L. Matlova, J. Bartl, L. Dvorska, I. Melicharek, and I. Pavlik. 2000. Findings of mycobacteria in insectivores and small rodents. Folia Microbiol. 45:147-152. [DOI] [PubMed] [Google Scholar]
- 18.Fischer, O., L. Matlova, L. Dvorska, P. Svastova, J. Bartl, I. Melicharek, R. T. Weston, and I. Pavlik. 2001. Diptera as vectors of mycobacterial infections in cattle and pigs. Med. Vet. Entomol. 15:208-211. [DOI] [PubMed] [Google Scholar]
- 19.Fischer, O. A., L. Matlova, J. Bartl, L. Dvorska, P. Svastova, R. Du Maine, I. Melicharek, M. Bartos, and I. Pavlik. 2003. Earthworms (Oligochaeta, Lumbricidae) and mycobacteria. Vet. Microbiol. 91:325-338. [DOI] [PubMed] [Google Scholar]
- 20.Fischer, O. A., L. Matlova, J. Dvorska, P. Svastova, and I. Pavlik. 2003. Nymphs of the Oriental cockroach, Blatta orientalis as passive vectors of causal agents of avian tuberculosis and paratuberculosis. Med. Vet. Entomol. 17:145-150. [DOI] [PubMed] [Google Scholar]
- 21.Fodstad, F. 1977. Tuberculin reactions in bulls and boars sensitized with atypical mycobacteria from sawdust. Acta Vet. Scand. 18:374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Framstad, T., and K. A. Rein. 2001. The use of peat to prevent diarrhoea after weaning. Int. Pig Top. 16:7-9. [Google Scholar]
- 23.Fuchs, B., J. Orda, J. Pres, and M. Muchowicz. 1995. The effect of feeding piglets up to the 100th day of their life with peat preparation on their growth and physiological and biochemical indices. Arch. Vet. Pol. 35:97-107. [PubMed] [Google Scholar]
- 24.Fuhrmann, C., and C. R. Lammler. 1997. Rhodococcus equi—causative agent of lymphadenitis in pig and cattle—relevance for meat production and processing. Fleischwirtschaft 77:840-842. (In German.) [Google Scholar]
- 25.Gardner, I. A., and D. W. Hird. 1989. Environmental source of mycobacteriosis in a California swine herd. Can. J. Vet. Res. 53:33-37. [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrero, C., J. Bernasconi, D. Burki, T. Bodmer, and A. Telenti. 1995. A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. J. Clin. Microbiol. 33:304-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horvathova, A., J. Kazda, J. Bartl, and I. Pavlik. 1997. Occurrence of conditionally pathogenic mycobacteria in life environment and their influence on living organism. Veterinarni Medicina 42:191-212. (In Slovak.) [PubMed] [Google Scholar]
- 28.Kazda, J. 2000. The ecology of the mycobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 29.Komijn, R. E., P. E. de Haas, M. M. Scneider, T. Eger, J. H. Nieuwenhuijs, R. J. van Den Hoek, D. Bakker, F. G. van Zijd Erveld, and D. van Soolingen. 1999. Prevalence of Mycobacterium avium in slaughtered pigs in The Netherlands and comparison of IS1245 restriction fragment length polymorphism patterns of porcine and human isolates. J. Clin. Microbiol. 37:1245-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozak, A., V. Vecerek, P. Chloupek, B. Tremlova, and M. Malena. 2003. Veterinary meat inspection of pig carcasses in the Czech Republic during the period of 1995-2002. Veterinarni Medicina 48:207-213. [Google Scholar]
- 31.Kubin, M., B. Burianova, L. Mezensky, M. Slosarek, and M. Turzova. 1986. Diagnosis of mycobacterial infections, p. 32-42. In J. Schindler, B. Tichacek, and V. Potuznik (ed.), Mikrobiologicke vysetrovaci metody, vol. 3. Avicenum, Prague, Czechoslovakia. (In Czech.) [Google Scholar]
- 32.Kunze, Z. M., S. Wall, R. Appelberg, M. T. Silva, F. Portaels, and J. J. McFadden. 1991. IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Mol. Microbiol. 5:2265-2272. [DOI] [PubMed] [Google Scholar]
- 33.Kunze, Z. M., F. Portaels, and J. J. McFadden. 1992. Biologically distinct subtypes of Mycobacterium avium differ in possession of insertion sequence IS901. J. Clin. Microbiol. 30:2366-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leinemann, H., H. Berner, H. Gerlach, H. Kalau, and J. Kosters. 1993. Mycobacteriosis on pigs due to Mycobacterium-avium-intracellulare. Tierärztl. Umsch. 48:713-718. (In German.) [Google Scholar]
- 35.Lenk, T., and A. Benda. 1989. Peat paste, a humic acid containing animal health agent for prevention and treatment of diarrhoea in calves. Monatshefte für Veterinärmedizin 44:563-565. (In German.) [Google Scholar]
- 36.Margolis, M. J., L. J. Hutchinson, K. B. Kephart, A. L. Hattel, R. H. Whitlock, and J. B. Payeur. 1994. Results of staining to confirm a diagnosis of swine mycobacteriosis made on the basis of gross examination. J. Am. Vet. Med. Assoc. 204:1571-1572. [PubMed] [Google Scholar]
- 37.Masaki, S., K. Shimizu, N. Cho, and T. Hirose. 1982. Isolation of mycobacteria from nodes of pigs and their environment. Nippon Juigaku Zasshi 44:213-221. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 38.Matlova, L., L. Dvorska, J. Bartl, M. Bartos, W. Y. Ayele, M. Alexa, and I. Pavlik. 2003. Mycobacteria isolated from the environment of pig farms in the Czech Republic during the years 1996 to 2002. Veterinarni Medicina 48:343-357. [Google Scholar]
- 39.Matlova, L., O. Fischer, J. Kazda, J. Kaustova, J. Bartl, A. Horvathova, and I. Pavlik. 1998. Occurrence of mycobacteria in invertebrates and poikilothermic animals and their role in man and other animals. Veterinarni Medicina 43:115-132. (In Czech.) [Google Scholar]
- 40.Matouskova, O., J. Chalupa, M. Cigler, and K. Hruska. 1992. Stat plus manual. Veterinary Research Institute, Brno, Czech Republic. (In Czech.)
- 41.Mijs, W., P. de Haas, R. Rossau, T. van der Laan, L. Rigouts, F. Portaels, and D. van Soolingen. 2002. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium to bird-type isolates and M. avium subsp. hominissuis for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 52:1505-1518. [DOI] [PubMed] [Google Scholar]
- 42.Monaghan, M. L., M. L. Doherty. J. D. Collins, J. F. Kazda, and P. J. Quinn. 1994. The tuberculin test. Vet. Microbiol. 40:111-124. [DOI] [PubMed] [Google Scholar]
- 43.Morita, Y., S. Maruyama, and Y. Katsube. 1994. Prevalence of atypical mycobacteriosis in slaughtered swine in Gunma prefecture and the serovars of the isolates. J. Vet. Med. Sci. 56:475-479. [DOI] [PubMed] [Google Scholar]
- 44.Morita, Y., M. Arai, O. Nomura, S. Maruyama, and Y. Katsube. 1994. Avian tuberculosis occurring in an imported pigeon and pathogenicity of the isolates. J. Vet. Med. Sci. 56:585-587. [DOI] [PubMed] [Google Scholar]
- 45.Nagai, R., S. Takewaki, A. Wada, K. Okuzumi, A. Tobita, and A. Ohkubo. 1990. Development of rapid detection method for mycobacteria using PCR. J. Med. Technol. 38:1247-1252. (In Japanese.) [PubMed] [Google Scholar]
- 46.Nishimori, K., M. Eguchi, Y. Nakaoka, Y. Onodera, T. Ito, and K. Tanaka. 1995. Distribution of IS901 in strains of Mycobacterium avium complex from swine by using IS901-detecting primers that discriminate between Mycobacterium avium and Mycobacterium intracellulare. J. Clin. Microbiol. 33:2102-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Offermann, U., T. Bodmer, L. Audige, and T. Jemmi. 1999. The prevalence of salmonella, yersinia and mycobacteria in slaughtered pigs in Switzerland. Schweiz. Arch. Tierheilkd. 141:509-515. (In German.) [PubMed] [Google Scholar]
- 48.Pavlik, I., L. Matlova, L. Dvorska, J. Bartl, L. Oktabcova, J. Docekal, and I. Parmova. 2003. Tuberculous lesions in pigs in the Czech Republic during 1990-1999: occurrence, causal factors and economic losses. Veterinarni Medicina 48:113-125. [Google Scholar]
- 49.Pavlik, I., P. Svastova, J. Bartl, L. Dvorska, and I. Rychlik. 2000. Relationship between IS901 in the Mycobacterium avium complex strains isolated from birds, animals, humans and environment and virulence for poultry. Clin. Diagn. Lab. Immunol. 7:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearson, C. W., L. A. Conner, and A. W. D. Leper. 1977. Tuberculin sensitivity of cattle inoculated with atypical mycobacteria isolated from cattle, feral pigs and trough water. Aust. Vet. J. 53:67-71. [DOI] [PubMed] [Google Scholar]
- 51.Ramasoota, P., N. Chansiripornchai, G. Kallenius, S. E. Hoffner, and S. B. Svenson. 2001. Comparison of Mycobacterium avium complex (MAC) strains from pigs and humans in Sweden by random amplified polymorphic DNA (RAPD) using standardized reagents. Vet. Microbiol. 78:251-259. [DOI] [PubMed] [Google Scholar]
- 52.Ritacco, V., K. Kremer, T. van Der Laan, J. E. Pijnenburg, P. E. W. de Haas, and D. van Soolingen. 1998. Use of IS901 and IS1245 in RFLP typing of Mycobacterium avium complex: relatedness among serovar reference strains, human and animal isolates. Int. J. Tuberc. Lung Dis. 2:242-251. [PubMed] [Google Scholar]
- 53.Roost, H., I. Dobberstein, G. Kuntsch, H. Berber, H. Tarder, A. Benda, and E. Helms. 1990. Use of a peat paste to prevent and control diarrhoea in intensively-reared piglets. Monatshefte für Veterinärmedizin 45:239-243. (In German.) [Google Scholar]
- 54.Songer, J. G., E. J. Bicknell, and C. O. Thoen. 1980. Epidemiological investigation of swine tuberculosis in Arizona. Can. J. Comp. Med. 44:115-120. [PMC free article] [PubMed] [Google Scholar]
- 55.Sûssland, Z., and V. Hrdinova. 1976. Use of rapid agglutination in the serotyping of the Mycobacterium avium-intracellulare complex. Vet. Med. (Prague) 21:209-213. (In Czech.) [PubMed] [Google Scholar]
- 56.Takai, S., N. Fukunaga, S. Ochiai, Y. Imai, W. Sasaki, S. Tsubaki, and T. Sekizaki. 1996. Identification of intermediately virulent Rhodococcus equi isolates from pigs. J. Clin. Microbiol. 34:1034-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorel, M. F., H. Huchzermeyer, R. Weiss, and J. J. Fontaine. 1997. Mycobacterium avium infections in animals. Vet. Res. 28:439-447. [PubMed] [Google Scholar]
- 58.Tuffley, R. E., J. H. Leggo, G. C. Simmons, and L. Tammemägi. 1973. Studies on the virulence of Mycobacterium intracellulare serotype VI for pigs. J. Comp. Pathol. 83:467-471. [DOI] [PubMed] [Google Scholar]
- 59.van Soolingen, D., J. Bauer, V. Ritacco, S. C. Leao, I. Pavlik, C. Vincent, N. Rastogi, A. Gori, T. Bodmer, D. Garzelli, and M. J. Garcia. 1998. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: proposal for standardization. J. Clin. Microbiol. 36:3051-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wayne, L. G., and G. P. Kubica. 1986. Family Mycobacteriaceae Chester 1897, 63AL. .In P. H. A. Sneath et al. (ed.), Bergey's manual of systematic bacteriology. Williams and Wilkins, Baltimore, Md.
- 61.Wilton, S., and D. Cousins. 1992. Detection and identification of multiple mycobacterial pathogens by DNA amplification in a single tube. PCR Methods Appl. 1:269-273. [DOI] [PubMed] [Google Scholar]
- 62.Windsor, R. S., D. S. Durrant, and K. J. Burn. 1984. Avian tuberculosis in pigs: Mycobacterium intracellulare infection in a breeding herd. Vet. Rec. 19:497-500. [DOI] [PubMed] [Google Scholar]
- 63.Wolinsky, E., and W. B. Schaefer. 1973. Proposed numbering scheme for mycobacterial serotypes by agglutination. Int. J. Syst. Bacteriol. 23:182-183. [Google Scholar]