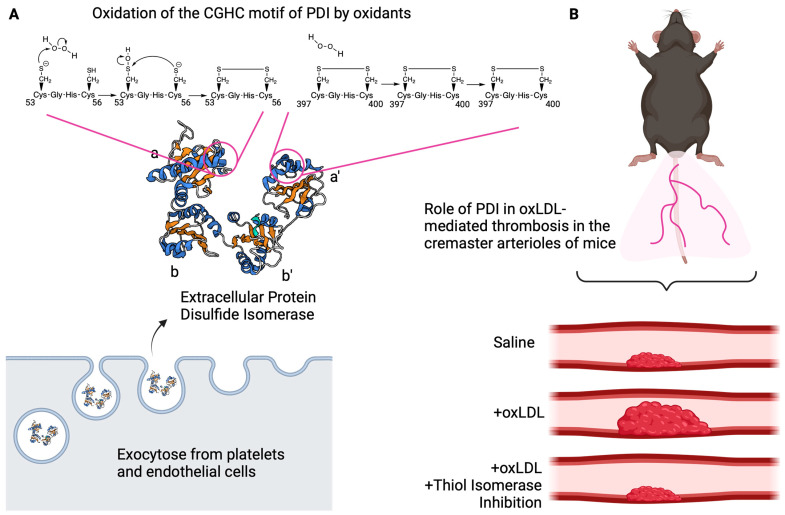
Figure 4.
Thiol isomerases link oxidative stress to thrombosis. (A) Protein disulfide isomerase (PDI) contains redox-active cysteines within CGHC motifs of the catalytic a and a′ domains. The CGHC motif of the a domain is sensitive to oxidation (sulfenylation) by peroxides, while the a′ domain is in an oxidized state and is less sensitive. PDI secreted from activated human vein endothelial cells and platelets contains a fraction of PDI that is sulfenylated. The mechanism of sulfenylation in the CGHC motif is drawn based on the more nucleophilic N-terminal cysteine; it is not clear which cysteine is being sulfenylated by peroxides; and it is also not clear how much of the fraction of exocytosed PDI is in the reduced or oxidized state. (B) In a laser-injury mouse model of cremaster arteriolar thrombosis, oxLDL infusion results in increased platelet accumulation compared to saline infusion. Inhibition of thiol isomerases with a PDI blocking monoclonal antibody RL90 prevents the enhanced thrombosis observed with oxLDL. Created with BioRender.com and accessed on 4 January 2024.