Abstract
In Spain, tuberculosis (TB) patterns are changing because of the recent increase in the number of cases among immigrants. To establish the composition of circulating Mycobacterium tuberculosis strains before the effects of foreign strains appear, this study focused on molecular characterization of 233 patient isolates using spoligotyping. The spoligotyping data were further analyzed using an international database, SpolDB4. The results obtained showed that the general features of the M. tuberculosis population in Spain are coherent with those of other European countries, with the Latin American and Mediterranean group, and with the Haarlem 3 and T1 families as the most prevalent genotypes. The Spanish isolates clustered mostly with genotypes which had previously been isolated in countries linked with Spain. We also describe and fully characterize two novel M. tuberculosis families, Madrid1 and Madrid2, which are specific to Spain-related settings. The data reported here provide a solid reference when monitoring changes in the composition of the M. tuberculosis population in Spain as a consequence of the increasing rate of TB in the foreign population.
The potential role of international social movements in modifying the patterns and transmission dynamics of tuberculosis (TB) has been studied in different countries (2, 8, 12). In Spain, the increase in the number of immigrants is still a recent phenomenon, which has become marked only in the last few years (10). Therefore, we thought it could be useful to obtain a baseline reference for the clonal composition of the circulating Mycobacterium tuberculosis strains in Madrid to assess the genetic structure of the global TB bacillus population in Madrid at a time when the effects of immigration were still quite moderate.
Traditionally, tuberculosis transmission could be understood only through contact tracing, using the classical methods of conventional epidemiology and the “stone-in-the-pond” principle (26). The advent of molecular epidemiology has shed light on the recent transmission rates of tuberculosis in the community, with the finding of higher transmission rates than suspected (25). The definition of “clusters,” groups of strains showing identical genetic characteristics, and their application to the assessment of recent tuberculosis transmission dynamics are the subjects of intense research. Furthermore, clusters are now widely accepted as representing phylogenetically significant information on the population structure of tubercle bacilli (and on their history) in a given setting (5). Indeed, the prevalences of different clones of M. tuberculosis vary from one region to another, leading to the interest in analyzing the worldwide population structure of tubercle bacilli (18).
The creation of international databases has revealed the clonal structure of M. tuberculosis populations in different geographic settings and has also defined superfamilies that are specific to certain countries (4, 14, 15). In this regard, the existence of an international database on spoligotyping (for spacer oligonucleotide typing), a PCR-based method that relies on a genetic locus called the direct repeat (11), which is highly polymorphic worldwide, has allowed some of the major superfamilies of M. tuberculosis to be described (6, 7).
The objective of this study was to describe the clonal composition of M. tuberculosis in Spain (in the Madrid area) in order to provide a reference which could be used to monitor potential changes in the genetic structure of the global population of the Spanish M. tuberculosis isolates. Indeed, the importation of TB cases to Spain through foreign-born patients from countries with a high TB burden may considerably affect circulating M. tuberculosis clones in the near future. Consequently, we decided to genotype 233 clinical isolates recruited during a 2-year period and to compare the results with those of an international database, SpolDB4, that previously contained some, although few, genetic data on M. tuberculosis clinical isolates in Spain.
MATERIALS AND METHODS
Strains.
All the M. tuberculosis strains were isolated at the Department of Clinical Microbiology and Infectious Diseases of the Gregorio Marañón Hospital in Madrid during a 2-year period (from January 2001 to December 2002). M. tuberculosis isolates were identified by using Accuprobe specific probes (Gene Probe, San Diego, Calif.). Only one M. tuberculosis strain per patient was selected for study. Madrid is divided into 11 health service areas, and we studied all the M. tuberculosis strains cultured from all the tuberculosis cases in area I, which is served by our hospital. This area is one of the biggest and most populated in Madrid, covering 1,142 km2 with 637,028 inhabitants. In Madrid, the numbers of cases of tuberculosis reported for the years 2001 and 2002 were 1,155 and 1,130, respectively; therefore, our sample represents ∼10% per year of the total TB cases in Madrid. The number of cases in Madrid represents a relevant part of the total number of cases in Spain (7,374 and 7,493 for the same years).
Clinical specimens were processed according to standard methods and inoculated in Lowenstein-Jensen slants and in MGIT (Becton Dickinson, Sparks, Md.) medium.
Molecular typing.
Spoligotyping was performed according to the manufacturer's instructions and the previously published procedure (11, 23).
IS6110 restriction fragment length polymorphism (RFLP) analysis was performed according to the international standardization guidelines (24).
Mycobacterial interspersed repetitive unit-variable-number tandem repeat (MIRU-VNTR) typing was performed as described elsewhere (22). MIRU-VNTR is a PCR-based typing method which assigns the number of tandem repeats in 12 independent loci (MIRUs) which are polymorphic in M. tuberculosis. The 12 PCRs specific for each MIRU locus were performed with primers and conditions detailed elsewhere (21). PCR products were separated by electrophoresis in MS-8 2% agarose gels (Pronadisa, Madrid, Spain), and the molecular sizes of the amplicons were calculated by comparing their mobilities with a 100-bp ladder marker (Gibco BRL), using the ChemiDoc system (Bio-Rad Laboratories, Richmond, Calif.) and Diversity database software (Bio-Rad). The number of repetitive units for each MIRU was calculated by means of the references included in the MIRU provisional web page (http://www.ibl.fr/mirus/mirus.html). The MIRU type consists of a 12-number code that indicates the number of tandem repeats found for each of the MIRU loci.
Phylogenetic comparisons between genotypes were performed using Bionumerics version 3.0 (Applied Maths, St. Marten-Latems, Belgium).
Database comparison and nomenclature.
The SpolDB4 Information System is an automated Access-based labeling and matching system for spoligotyping that will be described elsewhere (C. Delfino, N. Rastogi, and C. Sola, unpublished data). All of the data were also imported into the Bionumerics database format. The main improvement in SpolDB4 relative to SpolDB3 is that the comparison of newly introduced spoligotyping patterns to define new spoligotyping-based clusters with the database is now a fully automated process. SpolDB4 allows the simultaneous (i) introduction of any Excel file containing many hundreds to thousands of octal spoligotyping data (3), (ii) comparison of the new file with all of the spoligotypes contained in the database, and (iii) detection and labeling of new or preexisting matching orphan alleles based on the creation of an incremented shared type (ST) number if, and only if, at least two spoligotypes are identical. Before the introduction of the spoligotyping data set studied here, SpolDB4 contained a total of 26,384 isolates divided into 1,528 shared types and totalling 23,936 isolates plus 2,448 orphans. After the introduction of this file (n = 233), 15 new clusters were defined (ST1529 to ST1543), and SpolDB4 was reorganized into 24,156 isolates in shared types and 2,461 orphan alleles (for superfamily and family nomenclature, see http://www.cdc.gov/ncidod/eid/vol8no11/02-0125.htm) (6, 7). At the end of recruitment in April 2004, SpolDB4 included ∼40,000 isolates split into 1,939 shared types and ∼3,530 orphan profiles. A synthetic analysis of SpolDB4 is in progress and will be reported elsewhere. The building of SpolDB4 was made possible thanks to ∼80 participating laboratories and as many coinvestigators worldwide.
Epidemiological data.
For all cases with Madrid1 and Madrid2 M. tuberculosis isolates, epidemiological data were retrospectively collected from the Madrid Tuberculosis Register.
RESULTS
Analysis of M. tuberculosis genotypes. (i) General features.
Before the introduction of the Spain233 file, SpolDB4.0 contained a total of 26,384 isolates divided into 1,528 shared types and totaling 23,936 isolates plus 2,448 orphan isolates (http://www.cdc.gov/ncidod/EID/vol8no11/02-0125-Table.htm) (6, 7). After the introduction of this file (n = 233), 15 new clusters were defined, and SpolDB4 was reorganized into 24,156 isolates in shared types and 2,461 orphan alleles.
If we consider the 233 Spanish isolates alone, 71% were clustered (166 of 233), and the total number of orphan strains was 67 of 233 (29%) (Fig. 1 and Table 1). When we examined the Spanish isolates together with those in SpolDB4, 89% of the isolates were clustered (208 of 233) and 25 strains remained orphans (11%).
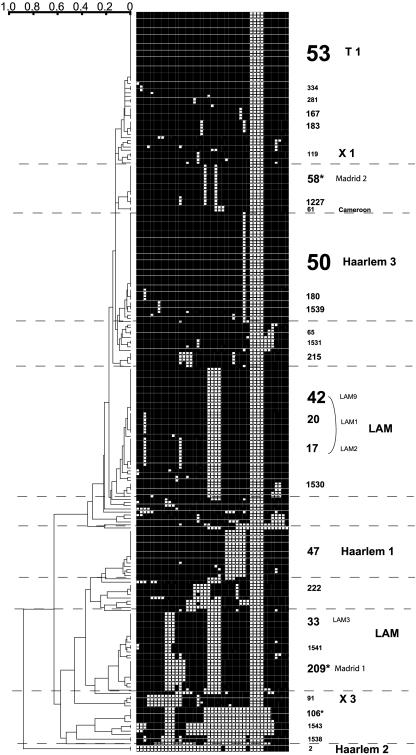
FIG. 1.
Similarity dendrogram obtained from the spoligotypes of the Spanish isolates in the spoligotyping results data set (n = 233). The scale on the left indicates the genetic distances among the M. tuberculosis strains (0 corresponds to identical patterns). On the right, the shared type numbers and family names, when applicable, for the clustered strains are shown. The text size is proportional to the sizes of the clusters. The asterisks indicate the shared types specific to Spain.
TABLE 1.
Full results of spoligotyping (in octal) and shared types designation after comparison with SpolDB4 (cf., Material and Methods)
| Key no. | Octal | STa | ||||
|---|---|---|---|---|---|---|
| ESP06200101009376 | 777777777760771 | 53 | ||||
| ESP06200101020433 | 777777777760771 | 53 | ||||
| ESP06200101023277 | 777777777760771 | 53 | ||||
| ESP06200101056013 | 777777777760771 | 53 | ||||
| ESP06200101085093 | 777777777760771 | 53 | ||||
| ESP06200202019099 | 777777777760771 | 53 | ||||
| ESP06200202024274 | 777777777760771 | 53 | ||||
| ESP06200202036688 | 777777777760771 | 53 | ||||
| ESP06200202039316 | 777777777760771 | 53 | ||||
| ESP06200202047949 | 777777777760771 | 53 | ||||
| ESP06200202048407 | 777777777760771 | 53 | ||||
| ESP06200202053247 | 777777777760771 | 53 | ||||
| ESP06200202053870 | 777777777760771 | 53 | ||||
| ESP06200202061308 | 777777777760771 | 53 | ||||
| ESP06200202074454 | 777777777760771 | 53 | ||||
| ESP06200202075361 | 777777777760771 | 53 | ||||
| ESP06200202075478 | 777777777760771 | 53 | ||||
| ESP06200202077762 | 777777777760771 | 53 | ||||
| ESP06200202117846 | 777777777760771 | 53 | ||||
| ESP06200202125364 | 777777777760771 | 53 | ||||
| ESP06200202129429 | 777777777760771 | 53 | ||||
| ESP06200202130066 | 777777777760771 | 53 | ||||
| ESP06200101098626 | 777777777760731 | 52 | ||||
| ESP06200101063856 | 777777777760711 | 78 | ||||
| ESP06200202024633 | 777777777760671 | 245 | ||||
| ESP06200101107485 | 777777777760631 | 888 | ||||
| ESP06200101071003 | 777777777760471 | 65 | ||||
| ESP06200101074946 | 777777777760471 | 65 | ||||
| ESP06200101128853 | 777777777740071 | 574 | ||||
| ESP06200101003631 | 777777777720771 | 50 | ||||
| ESP06200101007044 | 777777777720771 | 50 | ||||
| ESP06200101047262 | 777777777720771 | 50 | ||||
| ESP06200101062125 | 777777777720771 | 50 | ||||
| ESP06200101070061 | 777777777720771 | 50 | ||||
| ESP06200101079889 | 777777777720771 | 50 | ||||
| ESP06200101088714 | 777777777720771 | 50 | ||||
| ESP06200101105330 | 777777777720771 | 50 | ||||
| ESP06200101113340 | 777777777720771 | 50 | ||||
| ESP06200202005469 | 777777777720771 | 50 | ||||
| ESP06200202006561 | 777777777720771 | 50 | ||||
| ESP06200202008484 | 777777777720771 | 50 | ||||
| ESP06200202009964 | 777777777720771 | 50 | ||||
| ESP06200202082582 | 777777777720771 | 50 | ||||
| ESP06200202086379 | 777777777720771 | 50 | ||||
| ESP06200202096731 | 777777777720771 | 50 | ||||
| ESP06200202098161 | 777777777720771 | 50 | ||||
| ESP06200202103151 | 777777777720771 | 50 | ||||
| ESP06200202110601 | 777777777720771 | 50 | ||||
| ESP06200202111536 | 777777777720771 | 50 | ||||
| ESP06200202112395 | 777777777720771 | 50 | ||||
| ESP06200202127291 | 777777777720771 | 50 | ||||
| ESP06200202128087 | 777777777720771 | 50 | ||||
| ESP06200202132814 | 777777777720771 | 50 | ||||
| ESP06200101063541 | 777777777720771 | 1529* (a) | ||||
| ESP06200202010085 | 777777777660771 | 167 | ||||
| ESP06200202050232 | 777777777660771 | 167 | ||||
| ESP06200202051391 | 777777777660771 | 167 | ||||
| ESP06200202060878 | 777777777660771 | 167 | ||||
| ESP06200101077041 | 777777777320771 | 457 | ||||
| ESP0620000131 | 777777774020771 | 47 | ||||
| ESP06200101017492 | 777777774020771 | 47 | ||||
| ESP06200101035352 | 777777774020771 | 47 | ||||
| ESP06200101055858 | 777777774020771 | 47 | ||||
| ESP06200101070485 | 777777774020771 | 47 | ||||
| ESP06200101076401 | 777777774020771 | 47 | ||||
| ESP06200101079938 | 777777774020771 | 47 | ||||
| ESP06200202040612 | 777777774020771 | 47 | ||||
| ESP06200202050573 | 777777774020771 | 47 | ||||
| ESP06200202055049 | 777777774020771 | 47 | ||||
| ESP06200101089376 | 777777774020731 | 62 | ||||
| ESP06200202059245 | 777777764020771 | 45 | ||||
| ESP06200202061681 | 777777764020731 | 315 | ||||
| ESP06200202102117 | 777777754020771 | 883 | ||||
| ESP06200101092024 | 777777743760771 | 61 | ||||
| ESP06200202107683 | 777777743760771 | 61 | ||||
| ESP06200202010617 | 777777737760771 | 86 | ||||
| ESP06200202054595 | 777777717760731 | 513 | ||||
| ESP06200202004266 | 777777677760771 | 291 | ||||
| ESP06200101003070 | 777777607760771 | 42 | ||||
| ESP06200101008719 | 777777607760771 | 42 | ||||
| ESP06200101054345 | 777777607760771 | 42 | ||||
| ESP06200101055333 | 777777607760771 | 42 | ||||
| ESP06200101057188 | 777777607760771 | 42 | ||||
| ESP06200101083438 | 777777607760771 | 42 | ||||
| ESP06200101088055 | 777777607760771 | 42 | ||||
| ESP06200101091667 | 777777607760771 | 42 | ||||
| ESP06200202021339 | 777777607760771 | 42 | ||||
| ESP06200202064688 | 777777607760771 | 42 | ||||
| ESP06200202066225 | 777777607760771 | 42 | ||||
| ESP06200202067541 | 777777607760771 | 42 | ||||
| ESP06200202075028 | 777777607760771 | 42 | ||||
| ESP06200202095786 | 777777607760771 | 42 | ||||
| ESP06200202079111 | 777777607760711 | 1530* (b) | ||||
| ESP06200202079302 | 777777607760711 | 1530* (b) | ||||
| ESP06200202079886 | 777777607760711 | 1530* (b) | ||||
| ESP06200202092934 | 777777607760611 | 0 | ||||
| ESP06200202012793 | 777777607660771 | 891 | ||||
| ESP06200202045685 | 777777607400000 | 534 | ||||
| ESP06200202120710 | 777777604360771 | 0 | ||||
| ESP06200101000678 | 777777557760771 | 58 | ||||
| ESP06200101033212 | 777777557760771 | 58 | ||||
| ESP06200101091361 | 777777557760771 | 58 | ||||
| ESP06200202034006 | 777777557760771 | 58 | ||||
| ESP06200202047009 | 777777557760771 | 58 | ||||
| ESP06200202053146 | 777777557760771 | 58 | ||||
| ESP06200202054204 | 777777557760771 | 58 | ||||
| ESP06200202058421 | 777777557760771 | 58 | ||||
| ESP06200202079522 | 777777557760771 | 58 | ||||
| ESP06200202101807 | 777777557760771 | 58 | ||||
| ESP06200101092552 | 777777377760771 | 40 | ||||
| ESP06200101055575 | 777777377720771 | 183 | ||||
| ESP06200101085969 | 777777377720771 | 183 | ||||
| ESP06200101126302 | 777777377720771 | 183 | ||||
| ESP06200202124015 | 777777377720771 | 183 | ||||
| ESP06200202096784 | 777776777760771 | 119 | ||||
| ESP06200202102995 | 777776777760771 | 119 | ||||
| ESP06200202112069 | 777776777760601 | 137 | ||||
| ESP06200101058898 | 777776777760071 | 1531** | ||||
| ESP06200202034992 | 777776777760071 | 1531** | ||||
| ESP06200101105130 | 777776776160601 | 0 | ||||
| ESP06200101104707 | 777776775760771 | 1532* (c) | ||||
| ESP06200202018759 | 777775777760771 | 281 | ||||
| ESP06200202061753 | 777775777760771 | 281 | ||||
| ESP06200202079147 | 777775077560771 | 0 | ||||
| ESP0619970970436 | 777774077560771 | 222 | ||||
| ESP06200202058276 | 777774077560771 | 222 | ||||
| ESP06200202079188 | 777774077560771 | 222 | ||||
| ESP06200202089423 | 777766777760731 | 0 | ||||
| ESP06200202044915 | 777766777760071 | 0 | ||||
| ESP06200202114420 | 777763777720771 | 1533* (d) | ||||
| ESP06200202024348 | 777761007600771 | 0 | ||||
| ESP06200202118557 | 777760007760771 | 254 | ||||
| ESP06200101081257 | 777760007600771 | 0 | ||||
| ESP06200202075509 | 777737777700771 | 0 | ||||
| ESP06200202029726 | 777737774020771 | 218 | ||||
| ESP06200202053152 | 777737607760771 | 93 | ||||
| ESP06200101073952 | 777737557760771 | 1227 | ||||
| ESP06200202124576 | 777737557760771 | 1227 | ||||
| ESP06200202136436 | 777737557760771 | 1227 | ||||
| ESP06200101109124 | 777737477400001 | 0 | ||||
| ESP06200202016612 | 777723777360771 | 0 | ||||
| ESP06200202108205 | 777721607560731 | 1534* (e) | ||||
| ESP062001010130614 | 777703777360771 | 215 | ||||
| ESP06200202092941 | 777703777360771 | 215 | ||||
| ESP06200202111055 | 777703777360771 | 215 | ||||
| ESP06200101082979 | 777677607760771 | 770 | ||||
| ESP06200202039161 | 777667607760771 | 0 | ||||
| ESP06200202116155 | 777603405760471 | 136 | ||||
| ESP06200202053242 | 777577607760771 | 1535* (f) | ||||
| ESP06200202089621 | 776777707720771 | 0 | ||||
| ESP06200101101275 | 776377607760771 | 1536* (g) | ||||
| ESP06200101018901 | 776177777760771 | 156 | ||||
| ESP06200101014943 | 776177607760771 | 33 | ||||
| ESP06200101018215 | 776177607760771 | 33 | ||||
| ESP06200101024635 | 776177607760771 | 33 | ||||
| ESP06200101071603 | 776177607760771 | 33 | ||||
| ESP06200101104985 | 776177607760771 | 33 | ||||
| ESP06200101111774 | 776177607760771 | 33 | ||||
| ESP06200202044179 | 776177607760771 | 33 | ||||
| ESP06200202105761 | 776177607760771 | 33 | ||||
| ESP06200202110027 | 776177607760771 | 33 | ||||
| ESP06200202006621 | 776177607760731 | 130 | ||||
| ESP06200101015929 | 776177400000171 | 106 | ||||
| ESP06200101072339 | 776177400000171 | 106 | ||||
| ESP06200202028011 | 776177400000171 | 106 | ||||
| ESP06200202090934 | 776177400000171 | 106 | ||||
| ESP06200101101178 | 776177400000071 | 0 | ||||
| ESP06200101072015 | 776160000000071 | 105 | ||||
| ESP06200202037345 | 776137607760771 | 211 | ||||
| ESP06200202061812 | 776137607760731 | 1537* (h) | ||||
| ESP06200101022818 | 776137607760711 | 0 | ||||
| ESP06200101059429 | 776127400000171 | 0 | ||||
| ESP06200202016105 | 776037607760771 | 0 | ||||
| ESP06200101033451 | 776017607760771 | 209 | ||||
| ESP06200101113046 | 776017607760771 | 209 | ||||
| ESP06200202006451 | 776017607760771 | 209 | ||||
| ESP06200202017030 | 776017607760771 | 209 | ||||
| ESP06200202101855 | 776017607760771 | 209 | ||||
| ESP06200202112067 | 776017607760771 | 209 | ||||
| ESP06200202116152 | 776017607760771 | 209 | ||||
| ESP06200202039149 | 774137600020771 | 1538* (i) | ||||
| ESP06200202115484 | 774137600020771 | 1538* (i) | ||||
| ESP06200101050817 | 773777777720771 | 1539* (j) | ||||
| ESP06200101103661 | 773777777720771 | 1539* (j) | ||||
| ESP06200202005040 | 773777777720771 | 1539* (j) | ||||
| ESP06200202023798 | 773767777720771 | 0 | ||||
| ESP06200101066272 | 771777777760771 | 1069 | ||||
| ESP06200202124050 | 760001400000171 | 29 | ||||
| ESP06200101043848 | 757777777760771 | 154 | ||||
| ESP06200202075503 | 757777777320771 | 433 | ||||
| ESP06200101120091 | 757777607760611 | 0 | ||||
| ESP06200202111140 | 737737777760731 | 0 | ||||
| ESP06200101114557 | 700036777760771 | 91 | ||||
| ESP06200202016149 | 700036777760771 | 91 | ||||
| ESP06200202103753 | 700036776760771 | 0 | ||||
| ESP06200202089427 | 700036377760771 | 0 | ||||
| ESP06200202033204 | 677777777720771 | 180 | ||||
| ESP06200202059756 | 677777777720771 | 180 | ||||
| ESP06200202116151 | 677777777720771 | 180 | ||||
| ESP06200202136780 | 677777777720771 | 180 | ||||
| ESP06200101008451 | 677777607760771 | 20 | ||||
| ESP06200101098784 | 677777607760771 | 20 | ||||
| ESP06200101104470 | 677777607760771 | 20 | ||||
| ESP06200101113508 | 677777607760771 | 20 | ||||
| ESP06200202048022 | 677777607760771 | 20 | ||||
| ESP06200202115601 | 677777607760771 | 20 | ||||
| ESP06200202118380 | 677777607760771 | 20 | ||||
| ESP06200101002434 | 677737607760771 | 17 | ||||
| ESP06200101022121 | 677737607760771 | 17 | ||||
| ESP06200101022580 | 677737607760771 | 17 | ||||
| ESP06200101085378 | 677737607760771 | 17 | ||||
| ESP06200101093934 | 677737607760771 | 17 | ||||
| ESP06200202006486 | 677737607760771 | 17 | ||||
| ESP06200202029095 | 677737607760771 | 17 | ||||
| ESP06200202034480 | 677737607760771 | 17 | ||||
| ESP06200202121465 | 677737607760771 | 17 | ||||
| ESP06200202063828 | 577777777760771 | 334 | ||||
| ESP06200202101528 | 577777777760771 | 334 | ||||
| ESP06200202018681 | 437777774320731 | 0 | ||||
| ESP06200101124628 | 377777777760771 | 7 | ||||
| ESP06200202054848 | 377777607760771 | 177 | ||||
| ESP06200202098021 | 377377607760771 | 1540* (k) | ||||
| ESP06200202089426 | 376377777760771 | 884 | ||||
| ESP06200202035411 | 376377607760771 | 1541** | ||||
| ESP06200202049608 | 376377607760771 | 1541** | ||||
| ESP06200202110289 | 177776777760601 | 1542* (l) | ||||
| ESP06200101091768 | 176160000000071 | 0 | ||||
| ESP06200101055443 | 076160000000071 | 1543** | ||||
| ESP06200202115336 | 076160000000071 | 1543** | ||||
| ESP06200101078029 | 047777607760771 | 0 | ||||
| ESP06200202100200 | 037677777760771 | 1278 | ||||
| ESP06200101047458 | 000000004020771 | 2 | ||||
| ESP06200202035466 | 000000004020771 | 2 |
*, new shared type; **, new specific shared type (not found elsewhere); (a), match with a strain from Austria; (b), match with a strain from the United States; (c), match with a strain from the United States; (d) match with a strain from Austria; (e), match with a strain from the United States; (f), match with a strain from Brazil; (g), match with a strain from Brazil; (h), match with a strain from Italy; (i), match with a strain from Belgium (Moroccan immigrant); (j), match with a strain from Malaysia; (k), match with a strain from Indonesia; (l), match with a strain from the United States.
In terms of population genetics (Fig. 1), nine clonal complexes of seven or more strains were present in this study: ST50 (Haarlem 3 family; n = 24), ST 53 (superfamily T1; n = 22), ST42 (LAM9 family; n = 14), ST47 (Haarlem 1 family; n = 10), ST58 (n = 10), ST33 (LAM3; n = 9), ST17 (LAM2; n = 9), ST20 (LAM1; n = 7), and ST209 (n = 7).
(ii) Specific features.
Some shared types previously proposed as specific to Spain were also found in the Madrid spoligotyping data set (ST105, one new isolate, and ST106, four new isolates) (Table 1 and Fig. 1). Some isolates shared patterns with clusters which were already described in countries which have historical links with Spain, i.e., ST183 and ST222 (found in Peru and Mexico) and ST1227 and ST215 (found in Mexico and Texas) (16) (Table 1).
Of the 27 clusters found for the Spanish isolates, three had not been found in SpolDB4. For the time being, these clusters seem to be specific to the study setting and involve three microclusters of two strains each (Fig. 1): (i) ST 1541, which is close to ST33 (LAM3 family), and ST34 (S family); (ii) ST1543 which is close to ST106, already described in Spain (19); and (iii) ST1531.
After the inclusion of the 233 spoligotypes in SpolDB4, new isolates clustered with some previously orphan profiles, which were found in countries that could be considered historically linked to Spain. These include ST1538 (The Netherlands); ST1537 (Italy; Sicily); ST1535 and ST1536 (Brazil); ST1530, ST1532, ST1534, and ST1542 (United States); and ST1529 and ST1533 (Austria) (Table 1).
The main specific feature for the analysis of Spanish isolates is the presence of two highly prevalent genotypes that are potentially specific Spain-related genotypes. These are ST209 and ST58 (in this study, designated Madrid1 and Madrid2), with seven and nine isolates (Table 2), respectively. Both types had already been described in the international database with no identifiable trend concerning their origins, and now their distribution is statistically overrepresented in Latin countries, as shown in Table 2.
TABLE 2.
Characteristics of new Madrid 1 (ST209/LAM10) and Madrid 2 (ST58) families
BRA, Brazil; CUB, Cuba; DZA, Algeria; FXX, Metropolitan France; Bdx, Bordeaux; Par, Paris; ITA, Italy; NLD, The Netherlands; USA, United States of America; NYS, State of New York; Tx, Texas; Mi, Michigan; Mex, Mexico; ESP, Spain.
Isolate belonging to the principal genetic group 2 according to Sreevatsan et al. (20a), using katG-gyrA polymorphism.
Type U from the United States is different from the strains harboring identical seven copies in Spain.
Isolate belonging to the principal genetic group 3 according to Sreevatsan et al. (20a), using katG-gyrA polymorphism.
All strains from Spain highly similar.
ND, not done; NA, not available.
Brazilian immigrant; VNTR of this clinical isolate, 32533.
MIRU value of this clinical isolate, 223326153324.
MIRU value of this clinical isolate, 223326153324.
IPG, Institut Pasteur Guadeloupe; RIIPIA, Reseau International des Instituts Pasteur et Instituts Associes; RIVM, Rijk Institute of Veterinary Medicine.
Characterization of the Madrid1 and Madrid2 families.
An epidemiological survey did not reveal epilinks among most of the representatives of the Madrid1 or Madrid2 family. Only two cases in Madrid1 were clearly epidemiologically related (02112067 and 02116152) (Table 3). Madrid1 and Madrid2 strains were isolated from Spanish cases, except one case from Madrid1 (02101855), which corresponded to a 2-year-old Chinese child, and one case from Madrid2, a 25-year-old male who was born in Peru (Table 3).
TABLE 3.
General features of cases with Madrid1 and Madrid2 M. tuberculosis strains
| Sexa | Age (yr) | Nationality | Site of disease | Risk factor for TB | Epilinkb | M. tuberculosis strain |
|---|---|---|---|---|---|---|
| M | 42 | Spanish | Lung | HIV+, IVDUa | No | Madrid1 |
| M | 36 | Spanish | Ganglia | HIV+, IVDU | No | Madrid1 |
| M | 21 | Spanish | Lung | None | Yes | Madrid1 |
| M | 21 | Spanish | Lung | None | Yes | Madrid1 |
| M | 76 | Spanish | Lung | None | No | Madrid1 |
| M | 2 | Chinese | Lung | None | No | Madrid1 |
| M | 47 | Spanish | Lung | HIV+, prison | No | Madrid1 |
| F | 25 | Peruvian | Lung | None | No | Madrid2 |
| M | 65 | Spanish | Lung | None | No | Madrid2 |
| F | 27 | Spanish | Disseminated | None | No | Madrid2 |
| M | 34 | Spanish | Disseminated | HIV+ | No | Madrid2 |
| M | 21 | Spanish | Lung | None | No | Madrid2 |
| F | 74 | Spanish | Lung, nervous system | None | No | Madrid2 |
| M | 59 | Spanish | Lung | Alcoholism, IVDU | No | Madrid2 |
| M | 56 | Spanish | Lung | HIV+ | No | Madrid2 |
| M | 47 | Spanish | Lung | Alcoholism, IVDU | No | Madrid2 |
M, male; F, female.
Indicates whether case had epidemiological links with another case(s) within the Madrid1 or Madrid2 group.
HIV, human immunodeficiency virus; IVDU, intravenous drug user.
In order to characterize more precisely the Madrid1 and Madrid2 families, additional molecular typing by IS6110 RFLP and MIRU-VNTR was performed with the 16 isolates belonging to these families.
For ST209 (Madrid1), the RFLP analysis indicated that six isolates showed highly similar patterns, and the remaining isolate showed a pattern with lower similarity (Fig. 2). With regard to MIRUs, all seven isolates had identical genotypes (224326143323) (Fig. 2).
FIG. 2.
Similarity dendrogram obtained after combining the RFLP, spoligotyping, and VNTR-MIRU data for the isolates belonging to the Madrid1 (ST209) and Madrid2 (ST58) families. The scale on the left indicates the genetic distances among the M. tuberculosis strains (0 corresponds to identical patterns) taking RFLP, spoligotypes, and MIRU types together. On the right, the numbers for the isolates are shown.
For ST58 (Madrid2), the RFLP analysis indicated that most isolates showed highly similar patterns, with six to eight IS6110 copies (Fig. 2). With regard to MIRUs, all nine isolates showed highly similar MIRU patterns, with eight of the nine isolates showing an identical number of repeats in 11 of 12 MIRUs and differences only in MIRU 40 (Fig. 2).
The dendrogram obtained following the combined analysis of IS6110 RFLP, spoligotyping, and MIRU data showed that the isolates of the Madrid1 and Madrid2 families clustered in two similarity groups (Fig. 2).
DISCUSSION
In this study, we have defined the genetic structure of the population of circulating M. tuberculosis strains in Madrid based on a snapshot study of 233 clinical isolates. In our city, the numbers of cases of tuberculosis reported for the years 2001 and 2002 (1,155 and 1,130 cases, respectively) represent a relevant part of the total number of cases in Spain (7,374 and 7,493 for the same years). Therefore, we studied the clonal composition of M. tuberculosis strains in Madrid to obtain an idea of the clonal structure of tuberculosis in Spain at a time which coincides with marked increases in the number of immigrants coming to Spain and in the number of cases of tuberculosis that can be attributed to imported cases (from 6.7% in 1997 to 99 to 29.4% in 2002 and 2003). Different studies have analyzed the impact of immigration on the transmission dynamics of tuberculosis (2, 8, 12). However, most of these studies were performed in countries with a low prevalence of TB, a long history of immigration, and no snapshot of the situation before the immigrant population increased. Our study, on the other hand, was carried out during a transition period for TB transmission dynamics, which makes it possible to define a baseline reference for population genetic structure in Spain. This reference can now be used to precisely monitor the effect of immigration on the patterns and transmission dynamics of TB in the coming years.
In order to understand the genetic structure of the worldwide M. tuberculosis population and its evolution, the creation and development of international bacterial genotyping databases which gather and share M. tuberculosis typing patterns is of enormous value. These genotyping databases can help us to understand both the general and particular features of tuberculosis transmission worldwide, to detect casual transmission cases, and to define M. tuberculosis strains specific to different geographic settings which could be further chosen as reporter strains to monitor international TB routes. SpolDB4 allows us to perform this global analysis. Unfortunately, not all countries are equally represented in global databases, and prior to this study, the Spanish data included in the SpolDB3 database were limited. As a result, the general and specific features of the M. tuberculosis population in Spain were not easily appreciated. The inclusion of 233 spoligotyping patterns and some MIRU patterns from Spanish isolates in our study has increased our knowledge of this population.
If we compare Spanish spoligotyping patterns with all the data compiled in SpolDB4 from European isolates, we observe that the population structure found in our study is characteristic of a European country and shares the most prevalent European genotypes. For the Spanish isolates, ST50 (Haarlem 3 family) and ST53 (ill-defined T1) are the most prevalent genotypes (n = 24 and 22). The Latin American and Mediterranean (LAM) superfamily (20), with all its variants (ST17, ST20, ST42, and ST33), when taken as a whole, is the predominant superfamily (n = 39).
Several of our findings are consistent with a highly structured history of tuberculosis in Madrid, both historically and geographically. The inclusion of the Spanish data file revealed the following: (i) new isolates sharing patterns with genotypes previously proposed as specific to Spain or historically or geographically related countries; (ii) new isolates clustered with orphan profiles from countries which are related to Spain; and (iii) the most interesting finding, two new families of homogeneous genetic structures, which are likely to define Spain-specific clonal complexes of M. tuberculosis.
The first Spain-specific clade, ST209, is a new member of the LAM family of strains (designated LAM11 and Madrid1 in our study), and the second one is ST58 (designated Madrid2 in our study).
The distribution of ST209 (Madrid1) (Table 2) suggests Spanish phylogeographic specificity. Indeed, of 15 strains harboring this type, we found 10 in Spain, and the remaining isolates were found in Cuba, southwest France (Bordeaux), and Texas. Only one isolate belonging to ST209 was found in 2004 in the database of the Public Health Research Institute in the state of New York (J. Driscoll and B. Kreiwirth, personal communication). Most of the ST209 strains were shown to harbor identical or very similar 16- to 18-band IS6110 RFLPs (Fig. 2). In Spain, most of the strains harboring ST209 were also very similar by IS6110 RFLP, and they all had identical MIRUs (Fig. 2 and Table 2).
With regard to the other Spain-specific clade, ST58 (Madrid2), 71 strains harboring spoligotype ST58 were found in the database on 4 September 2003. They were distributed in Spain and other countries geographically or historically linked with Spain, including Brazil, Cuba, Algeria, France (two of which again originated in Bordeaux, in southwest France, near the Spanish border), French Guiana (from a Brazilian immigrant), Italy, The Netherlands, and the United States (the majority of which were found in Texas and New York [Latin-American cases]), and also in Venezuela and Mexico (Table 2). The distribution of this family, which is likely to belong to principal genetic group 3 (17), suggests a quite recent introduction and spread of a specific ancestral clone, perhaps from Austria or Italy. This clone may have spread and evolved specifically in Spanish-speaking countries and later spread to the United States.
A search of available IS6110 RFLP data for ST58 isolates from the SpolDB4 database resulted in various distinct profiles with five to eight bands (Table 2 and Fig. 2). In Spain, however, most of the strains harboring ST58 were also identical or highly similar by IS6110 RFLP and also showed highly similar or identical MIRU types. The MIRU type shared by four of nine ST58 isolates in Spain (223326153324) was also shared by three ST58 isolates (nonepidemiologically linked) from France and the United States (Michigan) (1, 9, 13).
A hypothetical scenario of evolution for this clonal complex suggests that ST44, which belongs to principal genetic group 3 (17), could be the origin of ST58 by the loss of spacer 19. This hypothesis relies on the finding of a high prevalence of ST44 in Austria, Italy, and the Czech Republic (39 out of 72 strains harboring this type in these three countries), countries whose history is closely linked with the former Spanish empire. However, at this stage, we cannot eliminate the possibility that the Madrid2 definition covers more than one clone and that convergence of spoligotypes occurred in two separate clones without a common ancestor, as demonstrated previously (27). However, the possibility of diverging IS6110 RFLP profiles within a single genetic ST58 background cannot be excluded.
It could be argued that our study is a snapshot of isolates obtained from a very limited geographic area and that the definition of two genotypes proposed to be Spain related must demonstrate that these genotypes are also found in other Spanish regions. Madrid1 and Madrid2 have also been found (two Madrid1 and one Madrid2 in 112 isolates) in a study which is currently being carried out in Almería (Andalucía, in southern Spain). Furthermore, Madrid1 and Madrid2 were found in another study in Segovia between 1995 and 1999 (2 Madrid1 and 4 Madrid2 in 96 strains analyzed). Finally, these genotypes were also found throughout Spain in the national M. tuberculosis database from the University of Zaragoza: 15 Madrid1 and 23 Madrid2 isolates from five and eight regions other than Madrid, respectively. It is also worth noting that Madrid1 and Madrid2 have been identified in 4 of 30 isolates from elderly people with tuberculosis (>65 years of age; probable reactivations) in Madrid (M. J. Rebollo, E. Palenque, S. Samper, F. Jaen, and M. J. Garcia, XI GEM Meeting, Torrelavega, Spain, poster, 2004), which suggests endemicity of these genotypes. Since the initial comparison made in September 2003 (15 ST209 and 71 ST58 isolates found), new submissions to SpolDB4 have increased the total number of clinical isolates harboring ST209 and ST58 in October 2004 to 24 and 95, respectively.
Altogether, these data demonstrate the highly historically and geographically structured history of tuberculosis in Madrid, a history that may be assessed both retrospectively and prospectively by genotyping using polymorphic markers, such as MIRU and spoligotyping, and by accessing worldwide genetic diversity databases. The distribution of isolates within 26,617 isolates in the database representing >100 countries suggests the high specificity of ST58 and ST209 clonal complexes for Spain and Spain-related areas.
Although robust, spoligotyping may be subject to misinterpretation, and the presence of typing artifacts should always be considered when interpreting results. MIRU-VNTR is presently the best complementary technique to demonstrate the clonality of some isolates, and it has been applied in this study to the ST58 and ST209 isolates. Congruence in the identity of MIRU markers makes it seem unlikely that ST58 and ST209 were produced by convergence and that they are not part of phylogenetic families. MIRU analysis will undoubtedly enable us to further discriminate the strains in ST58 and ST209 from other studies and may be helpful to discriminate between phylogenetic clusters and epiclusters. In parallel, contact tracing may reveal the presence of an epidemiological contact within these clusters. With the exception of two cases, an epidemiological survey in our study did not reveal epilinks among cases with Madrid1 and Madrid2 isolates. This rules out the possibility that these strains represent ongoing transmission within a specific population.
The existence of SpolDB4 and the public release of SpolDB3 should foster a further search for homogeneous clusters, as well as the description of their genetic characteristics, such as spoligotyping signature, most frequent VNTR and MIRU alleles, and IS6110 RFLP profiles. Recently, two new families of strains have been defined thanks to the retrospective analysis of IS6110 RFLP profiles. In Tanzania, McHugh et al. identified the Kilimanjaro 1 TB lineage, a characteristically Tanzanian clone, which may be a sublineage of the Central Asian 1-Delhi lineage (14). In the Philippines, Douglas et al. and Sola et al. described the Manila family, (also designated as the East African-Indian 2 genotype), which is part of the largest EAI superfamily (4, 6). Recently, the Cameroon family of TB bacilli was also identified (15). Undoubtedly, many more genetic families or lineages of TB bacilli, with as yet unknown specific pathogenicities, will be described in the near future, and these, together with Madrid1 and Madrid2, would be useful as reporter strains to explain the international routes of transmission of tuberculosis worldwide.
In conclusion, our study defines the general and specific features of the clonal composition of the M. tuberculosis population in Spain at a point in time when the immigrant population is increasing rapidly. Our data could therefore provide a useful reference for the composition of M. tuberculosis strains in a country before the influence of immigration on the population genetic structure becomes too important and erases historical traces of previous TB epidemics. These data will be used in forthcoming years as a reference for the real-time observation of the likely effects that a marked increase in immigration from countries with a high TB burden could have on the patterns and transmission dynamics of TB.
ESP0620020204491517777777777207711540* (k)ESP062002020050407737777777207711539* (j) ESP062002020237987737677777207710 ESP062001010662727717777777607711069 ESP0620020212405076000140000017129 ESP06200101043848757777777760771154 ESP06200202075503757777777320771433 ESP062001011200917577776077606110 ESP062002021111407377377777607310 ESP0620010111455770003677776077191 ESP0620020201614970003677776077191 ESP062002021037537000367767607710 ESP062002020894277000363777607710 ESP06200202033204677777777720771180 ESP06200202059756677777777720771180 ESP06200202116151677777777720771180 ESP06200202136780677777777720771180 ESP0620010100845167777760776077120 ESP0620010109878467777760776077120 ESP0620010110447067777760776077120 ESP0620010111350867777760776077120 ESP0620020204802267777760776077120 ESP0620020211560167777760776077120 ESP0620020211838067777760776077120 ESP0620010100243467773760776077117 ESP0620010102212167773760776077117 ESP0620010102258067773760776077117 ESP0620010108537867773760776077117 ESP0620010109393467773760776077117 ESP0620020200648667773760776077117 ESP0620020202909567773760776077117 ESP0620020203448067773760776077117 ESP0620020212146567773760776077117 ESP06200202063828577777777760771334 ESP06200202101528577777777760771334 ESP062002020186814377777743207310 ESP062001011246283777777777607717 ESP06200202054848377777607760771177 ESP062002020980213773776077607711540* (k) ESP06200202089426376377777760771884 ESP062002020354113763776077607711541** ESP062002020496083763776077607711541** ESP062002021102891777767777606011542* (l) ESP062001010917681761600000000710 ESP062001010554430761600000000711543** ESP062002021153360761600000000711543** ESP062001010780290477776077607710 ESP062002021002000376777777607711278 ESP062001010474580000000040207712 ESP062002020354660000000040207712
Acknowledgments
We are grateful to Jeff Driscoll, Barun Mathema, and Barry Kreiswirth for communication of unpublished genotyping information on ST58 and ST209 clinical isolates from the state of New York. We also thank Jeanne Maugein from Bordeaux, France, for communication of unpublished information on clinical isolates; Fernando Chaves and Pablo Carrero for data from Segovia, Spain; Elia Palenque and Maria Jesus Garcia for data from elderly patients in Spain; Sofia Samper and Carlos Martin for data from the national M. tuberculosis database from the University of Zaragoza; and Jesús Iñigo and Elena Rodriguez for the epidemiological survey of cases with Madrid1 and Madrid2 isolates in this study. We are also thankful to Sandra Andrés and Yolanda Paredes for their support with the genotyping and to Thomas O'Boyle for his revision of the English in this article.
This work has been partially financed by grants from Comunidad de Madrid (08.2/0029.1/01) and Fondo de Investigaciones Sanitarias (02/0882; 03/0654).
This research has been approved by the appropriate research review boards. No organization with a financial interest in the subject matter was involved in the manuscript. The authors have disclosed any conflict of interest related to this article.
REFERENCES
- 1.Cowan, L. S., L. Mosher, L. Diem, J. P. Massey, and J. T. Crawford. 2002. Variable-number tandem repeat typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110 by using mycobacterial interspersed repetitive units. J. Clin. Microbiol. 40:1592-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahle, U. R., P. Sandven, E. Heldal, and D. A. Caugant. 2001. Molecular epidemiology of Mycobacterium tuberculosis in Norway. J. Clin. Microbiol. 39:1802-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dale, J. W., D. Brittain, A. A. Cataldi, D. Cousins, J. T. Crawford, J. Driscoll, H. Heersma, T. Lillebaek, T. Quitugua, N. Rastogi, R. Skuce, C. Sola, D. van Soolingen, and V. Vincent. 2001. Spacer oligonucleotide typing of Mycobacterium tuberculosis: recommendations for standardized nomenclature. Int. J. Tuberc. Lung Dis. 5:216-219. [PubMed] [Google Scholar]
- 4.Douglas, J. T., L. Qian, J. C. Montoya, J. M. Musser, J. D. van Embden, D. van Soolingen, and K. Kremer. 2003. Characterization of the Manila family of Mycobacterium tuberculosis. J. Clin. Microbiol. 41:2723-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchene, V., S. Ferdinand, I. Filliol, J. F. Guégan, N. Rastogi, and C. Sola. 2004. Phylogenetic reconstruction of the Mycobacterium tuberculosis complex within four settings of the Caribbean region: tree comparative analysis and first appraisal on their phylogeography. Infect. Gen. Evol. 4:5-14. [DOI] [PubMed] [Google Scholar]
- 6.Filliol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valétudie, D. D. Anh, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. Haas, H. Heersma, G. Källenius, E. Kassa-Kelembho, T. Koivula, H. M. Ly, A. Makristathis, C. Mammina, G. Martin, P. Moström, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. Ngo Niobe Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. de Waard, C. Sola, and N. Rastogi. 2002. Global distribution of Mycobacterium tuberculosis spoligotypes. Emerg. Infect. Dis. 8:1347-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filliol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valetudie, D. A. Dang, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. H. Haas, H. Heersma, E. Kassa-Kelembho, M. L. Ho, A. Makristathis, C. Mammina, G. Martin, P. Mostrom, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. N. Niobe-Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. H. de Waard, C. Sola, and N. Rastogi. 2003. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J. Clin. Microbiol. 41:1963-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng, E., B. Kreiswirth, C. Driver, J. Li, J. Burzynski, P. DellaLatta, A. LaPaz, and N. W. Schluger. 2002. Changes in the transmission of tuberculosis in New York City from 1990 to 1999. N. Engl. J. Med. 346:1453-1458. [DOI] [PubMed] [Google Scholar]
- 9.Goguet de la Salmonière, Y. O., H. M. Li, G. Torrea, A. E. Bunschoten, J. D. A. van Embden, and B. Gicquel. 1997. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J. Clin. Microbiol. 35:2210-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupo de Trabajo de los Talleres de 2001 y 2002 de la Unidad de Investigación en Tuberculosis de Barcelona. 2003. Prevencion y control de las tuberculosis importadas. Med. Clin. (Barcelona) 121:549-557. [PubMed] [Google Scholar]
- 11.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. D. A. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lillebaek, T., A. B. Andersen, J. Bauer, A. Dirksen, S. Glismann, P. de Haas, and A. Kok-Jensen. 2001. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J. Clin. Microbiol. 39:855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazars, E., S. Lesjean, A. L. Banuls, M. Gilbert, V. Vincent, B. Gicquel, M. Tibayrenc, C. Locht, and P. Supply. 2001. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc. Natl. Acad. Sci. USA 98:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHugh, T. D., S. L. Batt, R. J. Shorten, R. D. Gosling, L. Uiso, and S. H. Gillespie. Mycobacterium tuberculosis lineage: a naming of the parts. Tuberculosis, in press. [DOI] [PubMed]
- 15.Ngo Niobe-Eyangoh, S., C. Kuaban, P. Sorlin, P. Cunin, J. Thonnon, C. Sola, N. Rastogi, V. Vincent, and M. C. Gutierrez. 2003. Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon. J. Clin. Microbiol. 41:2547-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quitugua, T. N., B. J. Seaworth, S. E. Weis, J. P. Taylor, J. S. Gillette, I. I. Rosas, K. C. Jost, Jr., D. M. Magee, and R. A. Cox. 2002. Transmission of drug-resistant tuberculosis in Texas and Mexico. J. Clin. Microbiol. 40:2716-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soini, H., X. Pan, A. Amin, E. A. Graviss, A. Siddiqui, and J. M. Musser. 2000. Characterization of Mycobacterium tuberculosis isolates from patients in Houston, Texas, by spoligotyping. J. Clin. Microbiol. 38:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sola, C., S. Ferdinand, C. Mammina, A. Nastasi, and N. Rastogi. 2001. Genetic diversity of Mycobacterium tuberculosis in Sicily based on spoligotyping and variable number of tandem DNA repeats and comparison with a spoligotyping database for population-based analysis. J. Clin. Microbiol. 39:1559-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sola, C., I. Filliol, C. Guttierez, I. Mokrousov, V. Vincent, and N. Rastogi. 2001. Spoligotype database of Mycobacterium tuberculosis: biogeographical distribution of shared types and epidemiological and phylogenetic perspectives. Emerg. Infect. Dis. 7:390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sola, C., I. Filliol, E. Legrand, I. Mokrousov, and N. Rastogi. 2001. Mycobacterium tuberculosis phylogeny reconstruction based on combined numerical analysis with IS1081, IS6110, VNTR and DR-based spoligotyping suggests the existence of two new phylogeographical clades. J. Mol. Evol. 53:680-689. [DOI] [PubMed] [Google Scholar]
- 21.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36:762-771. [DOI] [PubMed] [Google Scholar]
- 23.van Embden, J. D. A., T. van Gorkom, K. Kremer, R. Jansen, B. A. M. van der Zeijst, and L. M. Schouls. 2000. Genetic variation and evolutionary origin of the direct repeat locus of Mycobacterium tuberculosis complex bacteria. J. Bacteriol. 182:2393-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 26.Veen, J. 1992. Microepidemics of tuberculosis: the stone-in-the-pond principle. Tuberc. Lung Dis. 73:73-76. [DOI] [PubMed] [Google Scholar]
- 27.Warren, R. M., E. M. Streicher, S. L. Sampson, G. D. Van Der Spuy, M. Richardson, D. Nguyen, M. A. Behr, T. C. Victor, and P. D. van Helden. 2002. Microevolution of the direct repeat region of Mycobacterium tuberculosis: implications for interpretation of spoligotyping data. J. Clin. Microbiol. 40:4457-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]