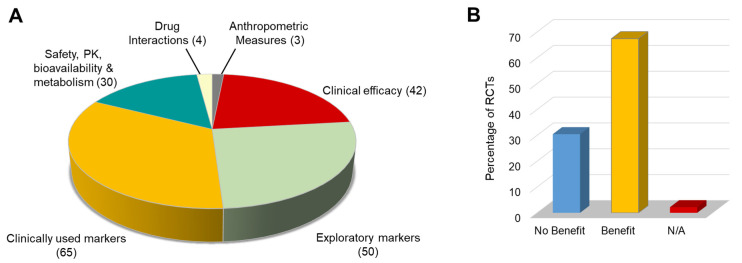
Figure 7.
Summary of the types of outcomes evaluated in RSV trials. (A) For all studies, including associated reanalyses, the primary outcomes were assigned into one of six categories to provide an overview of the extent of RSV clinical development. Where multiple primary outcomes have been listed in publications with no apparent hierarchy, the most robust endpoint has been used to classify the study where clinical efficacy > clinically used markers > exploratory markers. The figures in parentheses represent the number of studies in each category. (B) Proportion of RCTs and associated reanalyses that reported a benefit, or lack of benefit, against the primary outcomes. N/A means that the assessment of benefit was not applicable. Data presented relate to all references cited in Table 1 and Table S1.