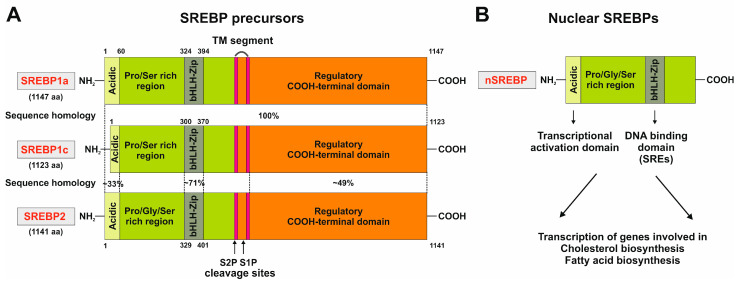
Figure 3.
Domain structure of the three SREBP family members SREBP1a, SREBP1c, and SREBP2. (A) The precursors of the three members of the SREBP family are comprised of three domains, namely, an NH2-terminal domain composed of an acidic domain, a proline and serine-rich stretch, and a basic helix-loop-helix-leucine zipper domain, two hydrophobic transmembrane segments separated by a short loop of 30 amino acids, and a carboxy-terminal regulatory region. This part of the figure was redrawn in modified and extended form from [26]. (B) The processed nuclear SREBP (nSREBP) contains only the N-terminal part of the precursor, composed of the acidic domain acting as a transcriptional activation domain and the basic helix-loop-helix-leucine zipper motif that binds sterol response elements (SREs) and acts as a DNA-binding domain. For more details see text.