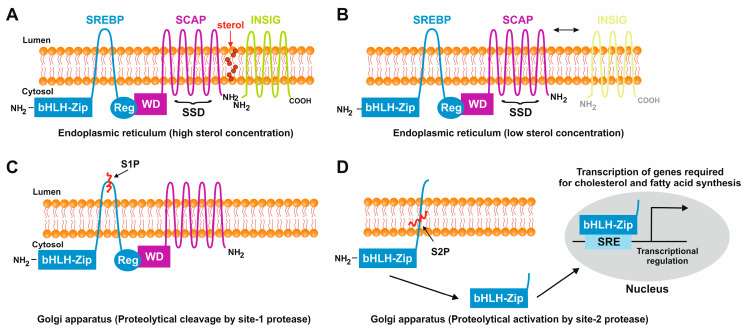
Figure 4.
Sterol-mediated proteolytic activation of SREBPs. (A) In conditions with abundant sterol content, SREBP, SCAP, and INSIG are anchored in the membranes of the endoplasmic reticulum. Under these conditions, SREBP has a hairpin orientation in which both the amino-terminal basic helix loop helix transcription factor motif and the carboxy-terminal regulatory domain are faced to the cytoplasm, while SCAP and INSIG bind to each other and form a hydrophobic cavity for sterol. (B) In conditions in which the sterol concentrations are low, the binding of SCAP and INSIG gets lost, and SCAP undergoes a conformational change that exposes a MELADL motif which is recognized by COPII. This protein serves as an escort protein, moving SCAP and bound SREBPs to the surface of the Golgi apparatus. (C) There, SREBP is proteolytically cleaved by the site-1 protease in the 30 amino acid stretch that links the transmembrane domains. (D) The site-2 protease cleaves SREBP within the membrane-spanning helix. This leads to the release of the carboxyl-terminal part of SREBP which contains the bHLH-Zip motif that shuttles to the nucleus where it activates the transcription of genes that are necessary for cholesterol and fatty acid biosynthesis.