Abstract
In veterinary medicine a complete blood cell count (CBC) cannot always be performed within 24 h as usually recommended, particularly for specimens shipped to a reference laboratory. This raises the question of the stability of the variables, especially in ethylenediamine tetra-acetic acid (EDTA) feline blood specimens, known to be prone to in vitro platelet aggregation. Citrate, theophylline, adenosine and dipyridamole (CTAD) has been reported to limit platelet aggregation in feline blood specimens. The aim of this study was to measure the stability of the haematological variables and the platelet aggregation score in EDTA and EDTA plus CTAD (EDCT) feline blood specimens during 48 h of storage at room temperature. Forty-six feline EDTA and EDCT blood specimens were analysed with a Sysmex XT-2000iV analyser, and the platelet count and score of platelet aggregation were estimated immediately and after 24 and 48 h of storage. A significant increase in mean corpuscular volume, haematocrit, reticulocyte and eosinophil counts, and a significant decrease in mean corpuscular haemoglobin concentration and monocyte count were observed. Haemoglobin, mean corpuscular haemoglobin, and red blood cell, white blood cell, neutrophil and lymphocyte counts remained stable. Changes in reticulocyte indexes with time (low fluorescence ratio, medium fluorescence ratio, high fluorescence ratio and immature reticulocyte fraction) were not significant. Changes were generally more pronounced in EDTA than in EDCT. Platelet aggregation decreased markedly in initially highly aggregated EDTA specimens, and increased slightly in initially non- or mildly-aggregated EDTA or EDCT specimens. Platelet counts increased and decreased, or remained stable, respectively. CTAD can reduce storage-induced changes of the haematological variables in feline samples, thus improving the reliability of a CBC and limiting clinical misinterpretations.
Introduction
In veterinary medicine, a complete blood cell count (CBC) is a common laboratory test used to assess the general health status of an animal. In practice, such laboratory tests can be performed routinely in veterinary clinics, as many in-house haematology analysers are now available. However, some clinics do not have adequate equipment, or an expert evaluation may be needed because the obtained results are erroneous, partial or require expertise. Thus, a CBC cannot always be performed within 24 h, as is usually recommended, and a delay of up to 48 h can occur when blood specimens have to be shipped to a laboratory. This raises the question of the stability of the different variables measured and of the validity of the results. Although much information is available about the stability of haematological variables in canine specimens, very little information is available about their stability in feline specimens.1 –5 Furthermore, in previous studies, (i) CBCs were performed in ethylenediamine tetra-acetic (EDTA) specimens, mostly by impedance technology and (ii) results were sometimes partial and preliminary as they did not follow international recommendations and were obtained from a small number of specimens. This might be owing to the difficulty of obtaining good EDTA specimens from cats. We recently reported that addition of citrate, theophylline, adenosine and dipyridamole (CTAD) to EDTA feline blood specimens (EDCT specimens) could improve the reliability of the CBC, especially of platelet and white blood cell (WBC) counts, by limiting the degree of aggregation. 6
The aim of this study was thus to report changes in haematological variables measured by the Sysmex XT-2000iV in feline blood specimens collected in K3-EDTA tubes with or without CTAD, and stored at room temperature for 24 and 48 h, thus mimicking the conditions of specimens sent to a referral laboratory over a weekend. The changes were investigated from statistical, analytical and clinical perspectives.
Materials and methods
Forty-six blood specimens were collected as part of a routine diagnostic evaluation or disease monitoring of cats admitted to the Hospital of the Toulouse Veterinary School. All owners had signed a consent form before sampling, accepting that the sample collected from their cat could be used for the study. Most blood specimens were collected without sedation (only three cats were sedated) from the jugular vein into a 5 ml K3-EDTA vacuum tube (Venoject; Terumo) with a 0.8 × 40 mm needle (Venosafe Mutisample; Terumo). Tubes were mixed gently by 10 inversions immediately after sample collection. All the tubes were filled correctly and did not show any macroscopic clots. Then, 3 ml of each specimen was pipetted into a tube containing CTAD (Vacuette; Greiner Bio-one GmbH), which was mixed by 10 inversions (EDCT tubes) then placed, along with the EDTA tube, for approximately 20 mins on a mechanical mixer (Speci Mix, CT06478, Drew) before being processed within 1 h of sampling (T0). After storage for 24 h at room temperature in the dark, the two tubes were gently mixed by 30 inversions and re-analysed (T24). The same procedure was repeated 24 h later (T48).
A blood film was prepared from each tube at T0, T24 and T48, air-dried and stained with modified May-Grünwald Giemsa (Aerospray Haematology Slide Stainer Cytocentrifuge 7150; Wescor), fixed, coverslip-mounted and stored in the dark. Smears were examined by the same trained veterinarian (FG) under light microscopy (BX60 microscope WH10X/22; Olympus) to estimate the platelet count and the degree of platelet aggregation. Platelet counts from the blood film (PLT-F) were estimated by calculating the mean number of platelets per oil-immersion (×1000) field (10 randomly selected fields in the monolayer area) multiplied by a pre-established factor of 15 × 109 cells/l. 7 The score of platelet aggregation from a blood film was estimated by a score according to Norman, as described previously.6,8 The mean of the 10 values was used as the score of platelet aggregation (SPA-F). EDTA specimens with a score <2 were considered arbitrarily as not or little aggregated (NLA) and specimens with a score ≥2 were considered as highly aggregated (HA).
A CBC was performed on each sample by the Sysmex XT-2000iV analyser (Sysmex), including WBC, red blood cell count (RBC), platelet count (PLT), haemoglobin (HGB), haematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), red blood cell distribution width as coefficient of variation (RDW-CV) and as percentage (RDW-SD) reticulocyte percentage (RET%) and count (RETc), grades of reticulocyte maturation as low, medium and high fluorescence ratios (LFR, MFR, HFR), the immature reticulocyte fraction (IRF) and the leukocyte differential count, with the corresponding species settings. The basophil counts are not reported as they were shown to be unreliable in feline specimens. 9 The PLT and RBC counts were determined by two different methods: flow cytometry and impedance cell counting (PLT-O, RBC-O and PLT-I, RBC-I). All counts measured in the EDCT tubes were multiplied by a factor (1.12) to compensate for the dilution with CTAD solution.
Quality control of the analyser was performed with the manufacturer’s control solution (Sysmex e-check L2). The within-run imprecision of the Sysmex XT-2000iV had been determined in a previous study. 10
The results of measurements at T0, T24 and T48 were compared by a two-way analysis of variance (ANOVA) test. The number of cases where the differences were higher than could be expected from within-run imprecision (2.77*CV) and the number of cases where they could account for a different classification of results according to recently published feline reference intervals (RIs) were also counted for some analytes when the RI were available. 11 Possible relationships between the results obtained at T0 and T24 or T48 for HCT, MCV and MCHC were investigated using Spearman’s correlation and Passing-Bablok’s agreement equations. The values measured at T0 for these variables were compared with the values calculated with the equations from the values measured at T24 or T48 by Student’s paired t-test after checking for variance homogeneity. Calculations were performed with an Excel spreadsheet, Analyse-It and Systat. As most distributions were significantly different from Gaussian, results are reported as the median and minimum–maximum range in parentheses.
Results
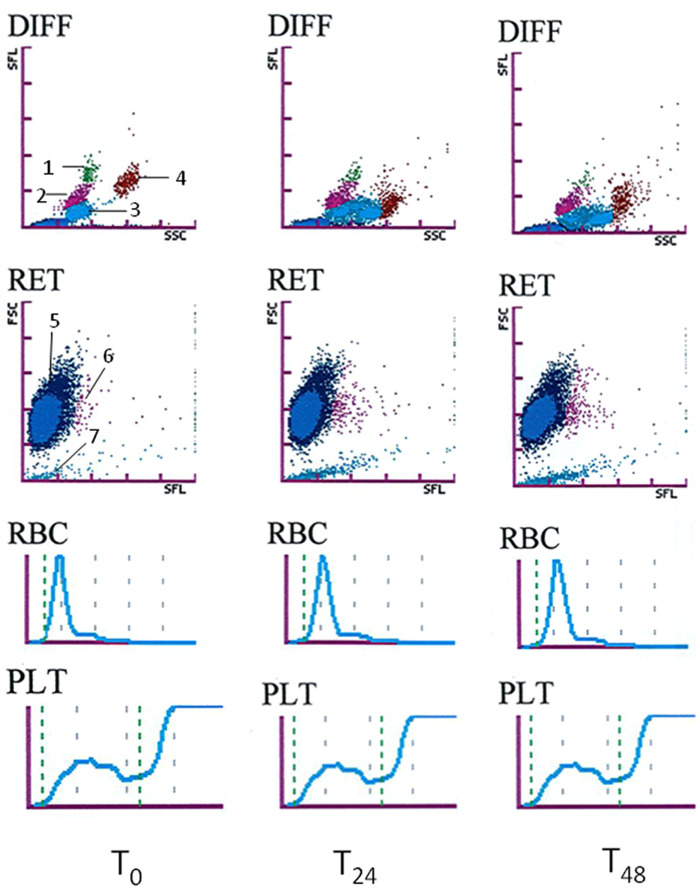
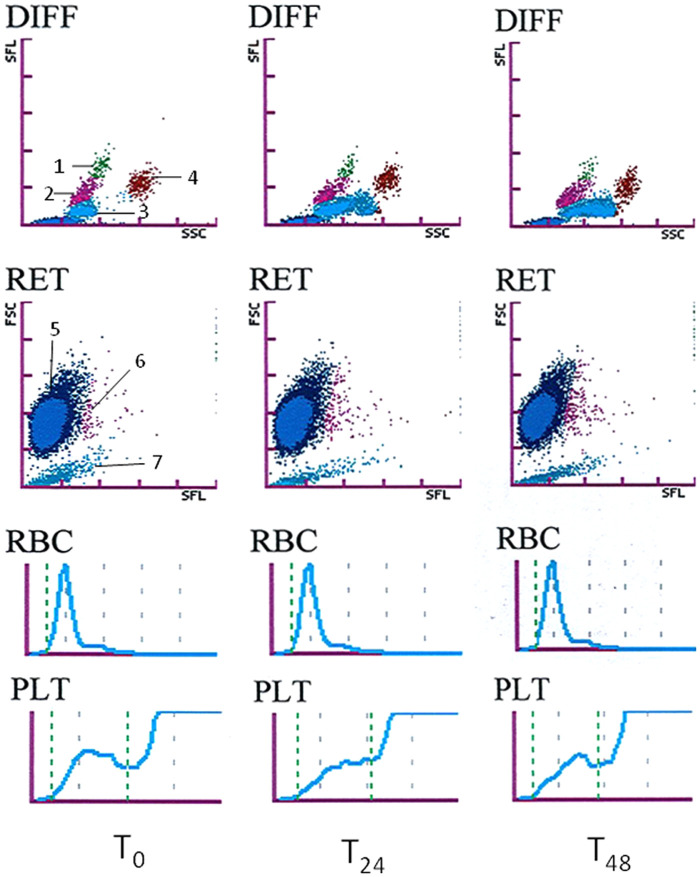
Forty-six feline blood specimens from 45 diseased cats and one healthy cat were analysed. Some results were unavailable because they were not given by the analyser owing to modifications caused by the illness of the cat or technical difficulties. For example, some differential counts were incomplete and one PLT-I had to be excluded because the cat presented with microcytosis causing an aberrant platelet count (~5.1012/l) by impedance technology. The final number of comparisons used for each variable is reported in Tables 1 and 2. Statistically significant effects of storage were observed for some variables, mostly in EDTA specimens. A typical case of scattergram changes from T0 to T48 in EDTA and EDCT is shown in Figures 1 and 2. In a high proportion of cases the differences were higher than could be explained by analytical variability. However, in many cases, the differences had little or no effect on the classification of results according to the RIs.
Table 1.
Changes in results of red and white blood cell variables obtained with Sysmex XT-2000iV on ethylenediamine tetra-acetic acid (EDTA) and EDTA plus citrate, theophylline, adenosine and dipyridamole (CTAD) (EDCT) feline blood samples during storage at room temperature in the dark for 24 (T24) and 48 (T48) h. For each variable, the following information is given: median (bold values), minimum–maximum range and percentage change (bold values) at each time (comparison by a two-way analysis of variance test, P<0.05; *,† or ‡: when the results for a variable are significantly different between T0 and T24, T0 and T48 or T24 and T48, respectively); number of cases where differences were higher than the maximal analytical variability with range of differences between parentheses; number of cases where classification according to reference intervals (RI) was different
| Variable | Unit | Anticoagulant | n | P | T0 | T24 | T48 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| RBC-I | 1012/l | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 7.87 (1.74/10.21) | 8.04 (1.65/10.52)* 25/44 (–0.56/0.11) 0/44 | +2.2% | 8.03 (1.69/10.47) † 21/44 (–0.63/0.12) 1/44 | +2.0% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 7.97 (1.76/10.39) | 8.03 (1.71/10.34) 4/42 (–0.26/0.15) 0/42 | +0.8% | 8.01 (1.73/10.39) 3/42 (–0.29/0.17) 0/42 | +0.5% | ||
| RBC-O | 1012/l | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 7.29 (1.55/9.59) | 7.37 (1.64/9.46) 16/44 (–0.28/0.19) 0/44 | +1.1% | 7.42 (0.81/9.67) 17/44 (–1.27/0.19) 0/44 | +1 .8% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 7.35 (1.56/9.68) | 7.31 (1.59/9.32) 13/42 (–0.29/0.14) 0/42 | –0.5% | 7.35 (1.53/9.72) 17/42 (–0.76/0.14) 0/42 | +0.0% | ||
| HCT | l/l | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 0.361 (0.130/0,480) | 0.414 (0.118/0.550)* 44/44 (–8.06/–0.30) 11/44 | +14.5% | 0.441 (0.106/0.573)†,‡ 44/44 (–11.55/–1.50) 15/44 | +22.2% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 0.377 (0.125/0.509) | 0.381 (0.118/0.500) 17/42 (–1.34/0.69) 1/42 | +1.0% | 0.390 (0.115/0.526) †,‡ 29/42 (–4.79/0.64) 1/42 | +3.4% | ||
| HGB | g/l | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 112.0 (30.0/144.0) | 112.5 (30.0/146.0)* 6/44 (–1.09/2.29) 0/44 | +0.5% | 113.0 (31.0/145.0)† 13/44 (–2.09/–2.28) 0/44 | +0.9% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.002 | 112.8 (30.2/144.1) | 112.3 (29.0/144.1) 2/42 (–3.61/2.39) 0/44 | –0.4% | 112.8 (30.2/145.2)‡ 2/42 (–2.18/2.39) 0/44 | +0.0% | ||
| MCV | fl | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 45.0 (35.1/78.2) | 49.1 (39.7/88.5)* 44/44 (–11.16/–1.69) 10/44 | +9.1% | 52.2 (41.6/92.9)†,‡ 44/44 (–15.36/–5.99) 17/44 | +16.0% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 46.5 (36.1/78.5) | 47.7 (37.3/89.5) 21/42 (–10.13/0.64) 0/42 | +2.6% | 48.4 (38.6/94.8)†,‡ 29/42 (–15.43/0.57) 4/42 | +4.1% | ||
| MCH | pg | EDTA | 44 | median (min/max) n >analytical dif (min/max) n classified differently / RI | 0.035 | 13.8 (10.8/18.4) | 13.8 (9.8/19.4)* 4/44 (–0.73/0.42) 0/44 | +0.0% | 13.8 (9.7/18.9) 7/44 (–0.83/0.36) 0/44 | +0.0% |
| EDCT | 42 | median (min/max) n >analytical dif (min/max) n classified differently / RI | 0.027 | 13.8 (10.4/17.7) | 13.6 (10.2/18.3)* 1/42 (–0.16/0.38) 0/42 | –1.5% | 13.8 (9.9/18.1)‡ 1/42 (–0.24/0.38) 0/42 | +0.0% | ||
|
| ||||||||||
| MCHC | g/l | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 306 (235/331) | 265.5 (219/292)* 44/44 (–53.58/–2.57) 9/44 | –13.2% | 251(204/292)†,‡ 44/44 (–66.58/–23.84) 25/44 | –18.0% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 290 (226/314) | 288 (204/325) 11/42 (–15.11/9.57) 0/42 | –0.7% | 286 (190/315)†,‡ 19/42 (–36.56/9.48) 1/42 | –1.4% | ||
| RDW-SD | fl | EDTA | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 30.6 (27.0/71.8) | 34.6 (29.3/72.0)* 42/43 (–6.99/1.19) ND | +13.1% | 38.0 (30.6/75)†,‡ 41/43 (–15.31/0.75) ND | +24.2% |
| EDCT | 41 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 31.1 (27.0/70.2) | 33.2 (28.3/78.5)* 39/41 (–6.94/0.50) ND | +6.8% | 35.5 (30.0/74.5)†,‡ 41/41 (–15.33/0.50) ND | +14.2% | ||
| RDW-CV | % | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.017 | 21.75 (18.2/30.2) | 21.30 (17.8/26.7)* 31/44 (–3.83/0.21) 4/44 | –2.1% | 21.90 (18.9/25.6)‡ 35/44 (–4.24/0.31) 3/44 | +0.7% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 20.9 (17.1/28.6) | 22.0 (18.3/29.3)* 39/42 (–2.55/0.31) 8/42 | +5.3% | 23.3 (19.2/28.0)†,‡ 42/42 (–6.05/0.26) 13/42 | +11.5% | ||
| RETc | 109/l | EDTA | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 375 (142/2692) | 522 (196/3371)* 36/43 (–474.80/106.99) ND | +39.2% | 641 (218/3366)†,‡ 39/43 (–520.32/41.59) ND | +70.9% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 357 (11/2394) | 440 (11/3050)* 29/42 (–430.0/91.8) ND | +23.3% | 505 (20/3276)†,‡ 40/42 (–719.95/32/42) ND | +41.5% | ||
| RET% | % | EDTA | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.001 | 0.53 (0.17/15.47) | 0.70 (0.23/20.43)* 33/43 (–3.37/0.80) ND | +32.1% | 0.80 (0.31/19.92)† 38/43 (–2.86/0.07) ND | +50.9% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.001 | 0.48 (0.05/13.57) | 0.55 (0.05/17.85) 27/42 (–2.89/0.08) ND | +14.6% | 0.73 (0.09/18.93)† 40/42 (–3.97/0.04) ND | +52.1% | ||
| LFR | % | EDTA | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 94.1 (68.9/100.0) | 94.3 (67.4/98.6) 0/43 (6.98/23.24) ND | +0.2% | 94.1 (68.7/98.9) 0/43 (4.65/23.16) ND | +0.0% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 93.7 (61.7/100.0) | 94.3 (68.4/98.1) 0/42 (8.00/22.83) ND | +0.6% | 94.2 (75.1/99.2) 0/42 (1.30/23.38) ND | +0.5% | ||
| MFR | % | EDTA | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 4.6 (0.0/20.8) | 4.3 (1.0/23.4) 14/43 (–2.70/12.15) ND | –6.5% | 5.0 (0.0/22.0) 19/43 (–7.40/13.55) ND | +8.7% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 4.7 (0.0/25.4) | 5.1 (0.0/22.5) 11/42 (–7.03/15.11) ND | +8.5% | 4.85 (0.0/19.1) 14/42 (–14.30/11.71)ND | +3.2% | ||
| HFR | % | EDTA | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 1.3 (0.0/10.3) | 0.8 (0.0/9.2) 11/43 (–2.70/9.94) ND | –38.5% | 0.9 (0.0/9.3) 11/43 (–2.30/10.04) ND | –30.8% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 1.0 (0.0/12.9) | 0.95 (0.0/12.5) 8/42 (–12.50/10.03) ND | +5.0% | (0.0/5.8) 10/42 (–2.90/6.73) ND | +0.0% | ||
| IRF | % | EDTA | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 5.9 (0.0/31.1) | 5.7 (1.4/32.6) 11/43 (–4.19/21.42) ND | –3.4% | 5.9 (30.2/1.1) 14/43 (–8.03/22.72) ND | +0.0% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently / RI | NS | 6.4 (0.0/38.3) | 5.7 (1.9/31.6) 12/42 (–12.50/21.52) ND | –10.9% | 5.7 (0.8/24.9) 11/42 (–14.30/14.82) ND | –10.9% | ||
| WBC | 109/l | EDTA | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 10.14 (3.82/79.69) | 10.28 (4.08/80.03) 5/44 (–0.41/2.75) 0/44 | +1.4% | 10.23 (3.91/80.58) 9/44 (–1.40/2.20) 0/44 | +0.9% |
| EDCT | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 9.68 (3.73/77.26) | 9.77 (3.93/77.61) 2/42 (0.78/2.65) 0/42 | +0.9% | 9.79 (3.80/78.96) 3/42 (–0.38/1.30) 0/42 | +1.1% | ||
| Neutrophils | 109/l | EDTA | 38 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 6.21 (1.16/24.85) | 6.32 (0.76/25.60) 17/38 (–0.65/2.75) 0/38 | +1.8% | 6.16 (1.87/25.04) 20/38 (–3.53/2.20) 2/38 | –0.8% |
| EDCT | 38 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.015 | 5.72 (0.85/24.05) | 5.86 (0.92/23.94)* 16/38 (–1.00/2.65) 1/38 | +2.5% | 5.86 (1.53/24.79) 17/38 (–0.97/1.30) 2/38 | +2.5% | ||
| Lymphocytes | 109/l | EDTA | 38 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 1.92 (0.31/7.35) | 1.69 (0.24/6.65) 27/38 (–2.17/0.29) 2/38 | –12.0% | 1.58 (0.12/6.36) 17/38 (–1.96/0.86) 3/38 | –17.7% |
| EDCT | 39 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 1.82 (0.35/7.47) | 1.49 (0.23/7.55) 12/39 (–1.32/1.18) 3/39 | –18.1% | 1.78 (0.11/7.29) 13/39 (–1.59/1.08) 4/39 | –2.2% | ||
| Monocytes | 109/l | EDTA | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.001 | 0.36 (0.05/6.99) | 0.29 (0.05/3.64) 3/42 (–0.56/0.26) 0/42 | –19.4% | 0.18 (0.03/1.63)† 25/42 (–2.57/0.11) 1/42 | –50.0% |
| EDCT | 41 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.005 | 0.35 (0.04/6.06) | 0.31 (0.04/3.08) 1/41 (–0.56/0.63) 0/41 | –11.4% | 0.26 (0.03/1.82)† 15/41 (–1.82/0.36) 0/41 | –25.7% | ||
| Eosinophils | 109/l | EDTA | 41 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 0.34 (0.03/2.37) | 0.42 (0.11/2.34)* 19/41 (–0.23/0.43) 2/41 | +23.5% | 0.45 (0.10/2.37)† 26/41 (–0.27/0.46) 2/41 | +32.4% |
| EDCT | 40 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 0.35 (0.01/2.38) | 0.42 (0.07/0.22) 11/40 (–0.44/0.30) 3/40 | +20.0% | 0.41 (0.07/2.27) 12/40 (–0.50/0.35) 3/40 | +17.1% | ||
HFR = high fluorescence ratio; HGB = haemoglobin; IRF = immature reticulocyte fraction; LFR = fluorescence ratio; MCH = mean corpuscular haemoglobin; MCHC = mean corpuscular haemoglobin concentration; MCV = mean corpuscular volume; MFR = medium fluorescence ratio; ND = not determined (RI not available for this variable 11 ); NS = not significantly different; RBC = red blood cell; RDW-CV = red blood cell distribution width as coefficient of variation; RDW-SD = red blood cell distribution width as percentage; RET% = reticulocyte percentage; RETc = reticulocyte count; RI = reference interval; WBC = white blood cell
Table 2.
Changes in results of platelet (PLT) count and degree of aggregation obtained with Sysmex XT-2000iV (PLT-I and PLT-O) or by a semi-quantitative estimation on a blood smear [PLT-F and score of platelet aggregation (SPA-F)] on ethylediamine tetra-acetic acid (EDTA) and EDTA plus citrate, theophylline, adenosine and dipyridamole (CTAD) (EDCT) feline blood samples during storage at room temperature in the dark for 24 (T24) and 48 (T48) h. For each variable, the following information is given: median (bold values), minimum–maximum range and percentage change (bold values) at each time (comparison by a two-way analysis of variance test, P<0.05;*,† or‡: when the results for a variable are significantly different between T0 and T24, T0 and T48, or T24 and T48, respectively); number of cases where differences were higher than the maximal analytical variability with range of differences between parentheses; number of cases where classification according to reference intervals was different
| Variable | Unit | Anticoagulant | n | P | T0 | T24 | T48 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PLT-I | 109/l | EDTA | All | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.004 | 126 (1/345) | 120 (11/310) 34/43 (–133.5/17.6) 5/43 | –4.8% | 154 (15/346)‡ 30/43 (–206.1/28.6) 4/43 | +22.2% |
| HA | 10 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 20 (1/111) | 107 (34/203)* 10/10 (–133.5/–22.5) 4/10 | +535.0% | 147 (30/306)†,‡ 10/10 (–206.1/–28.9) 5/10 | +735.0% | |||
| NLA | 33 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 170 (4/345) | 137 (11/310)* 24/33 (–126.8/17.6) 1/33 | –19.4% | 167 (15/346)†,‡ 20/33 (–66.8/28.6) 0/33 | –1.8% | |||
| EDCT | All | 41 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 166 (3/338) | 89 (9/251)* 39/41 (–166.36/2.57) 7/41 | –46.4% | 117 (7/270)†,‡ 38/41 (–155.20/6.31) 3/41 | –29.5% | ||
| PLT-O | 109/l | EDTA | All | 43 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 242 (24/518) | 251 (51/500) 21/43 (–231.8/32.2) 4/43 | +3.7% | 272 (79/588)†,‡ 25/43 (–288.7/31.4) 4/43 | +12.4% |
| HA | 10 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 103 (24/242) | 232 (113/370)* 9/10 (–231.8/0.3) 4/10 | +25.2% | 268 (145/392)† 10/10 (–288.7/–10.9) 4/10 | +60.2% | |||
| NLA | 33 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.001 | 269 (42/518) | 252 (51/500) 12/33 (–154.8/32.2) 0/33 | –6.3% | 278 (79/588)†,‡ 14/33 (–172.8/31.4) 0/33 | +3.4% | |||
| EDCT | All | 42 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.001 | 249 (28/514) | 241 (52/463)* 17/42 (–59.08/34.66) 2/42 | –3.2% | 241 (93/500)‡ 10/42 (–170.38/36.40) 2/42 | –3.2% | ||
| PLT-F | 109/l | EDTA | All | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 277 (9/638) | 276 (9/739) ND ND | –0.4% | 296 (6/791) ND ND | +6.9% |
| HA | 11 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.019 | 120 (36/358) | 199 (128/696)* ND ND | +65.8% | 261 (124/621)† ND ND | +217.5% | |||
| NLA | 33 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 309 (9/638) | 289 (9/739) ND ND | –6.5% | 309 (6/791) ND ND | +0.0% | |||
| EDCT | All | 46 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | 0.006 | 288 (6/810) | 325 (13/691)* ND ND | +12.9% | 333 (13/886)† ND ND | +15.6% | ||
| SPA-F | /5 | EDTA | All | 44 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | NS | 0.8 (0.0/4.3) | 1.3 (0.2/2.9) ND ND | +62.5% | 1.1 (0.3/2.0) ND ND | +37.5% |
| HA | 11 | Median (min/max) n >analytical diff. (min/max) n classified differently/ RI | <0.001 | 3.2 (2.5/4.3) | 2.3 (1.4/2.9)* ND ND | –28.1% | (0.6/3.1)† ND ND | –37.5% | |||
| NLA | 33 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 0.6 (0.0/1.4) | 1.1 (0.2/2.8)* ND ND | +83.3% | 1.0 (0.2/3.1)† ND ND | +66.7% | |||
| EDCT | All | 46 | Median (min/max) n> analytical diff (min/max) n classified differently/ RI | <0.001 | 0.5 (0.0/2.8) | 0.8 (0.3/2.5)* ND ND | +60.0% | 0.8 (0.3/2.0)† ND ND | +60.0% |
HA = highly aggregated; ND = not determined (RI not available for this 11 ); NLA: not or little aggregated; NS = not significantly different; PLT-F = platelet count estimated from the blood film; PLT-I = platelet count estimated by impedance technology; PLT-O = platelet count estimated by optical technology; RI = reference interval; SPA-F = score of platelet aggregation estimated from the blood film
Figure 1.
Classical example of the changes in white blood cell differential (DIFF), reticulocyte (RET) and platelet (PLT-O) scattergrams (optical measurement), red blood cell (RBC) and platelet (PLT) histograms (impedance measurement) in the ethylenediamine tetra-acetic acid (EDTA) blood sample from a cat at T0 and after storage at room temperature in the dark for 24 (T24) and 48 (T48) h. Description of the dot plot: 1= monocytes, 2 = lymphocytes, 3 = neutrophils, 4 = eosinophils, 5 = red blood cells, 6 = reticulocytes and 7 = platelets
Figure 2.
Classical example of the changes in white blood cell differential (DIFF), reticulocyte (RET) and platelet (PLT-O) scattergrams (optical measurement), red blood cell (RBC) and platelet (PLT) histograms (impedance measurement) in the ethylenediamine tetra-acetic acid plus citrate, theophylline, adenosine and dipyridamole (EDCT) blood sample from a cat (the same one as in Figure 1) at T0 and after storage at room temperature in the dark for 24 (T24) and 48 (T48) h. Description of the dot plot: 1 = monocytes, 2 = lymphocytes, 3 = neutrophils, 4 = eosinophils, 5 = red blood cells, 6 = reticulocytes and 7 = platelets
RBC
In EDTA specimens, HCT and MCV increased gradually and markedly during the 48 h of storage, causing clinical misclassification of 15/44 and 17/44 cases, respectively, at T48 (+22.2% and +16.0%). In addition, MCHC decreased and 25/44 specimens were classified differently, according to the RI at T48. Other RBC variables showed no or only slight statistical differences, with no or very few misclassifications of results according to the RI, except for RDW-SD, which increased during the 48 h of storage (+24.2% at T48). On the scattergrams, the RBC dot plots showed a moderate shift to the top in 34/44 cases.
In EDCT specimens, the changes for RBC variables and scattergrams were similar, but less pronounced, than in EDTA specimens. For example, HCT and MCV increased significantly only at T48 (+3.4% and +4.1%), and only 1/42 and 4/42 specimens were classified differently according to the RI at T48. MCHC decreased less than in EDTA, and RDW-CV and RDW-SD increased gradually during storage. RBC-O, RBC-I, HGB and MCH also showed no or only slight statistical differences during storage, and no specimen was classified differently according to the RI.
No significant difference was observed between the values measured at T0 and the calculated T24 and T48 results obtained for HCT, MCV and MCHC (Table 3). The calculated values gave no or very few misclassifications according to the RI.
Table 3.
Estimation of haematological results at T0 from results obtained after storage at room temperature in the dark for 24 (T24) and 48 h (T48) in ethylenediamine tetra-acetic acid (EDTA) and EDTA plus citrate, theophylline, adenosine and dipyridamole (CTAD) (EDCT) feline blood specimens. r is Spearman’s coefficient of correlation between values at T0 and T24 or T48. The equation used for estimation was Passing Bablok’s equation; np ≠T0: number of cases where classification according to reference intervals was different in values estimated by the Passing Bablok’s equation and values measured at T0; n24 or n48 ≠T0: number of cases where classification according to reference intervals was different between the values observed at T0 and T24 or T48
| Variable | Unit | Anticoagulant | T24 vs T0 |
T48 vs T0 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r T24 vs T0 | Equation used for estimation | np≠T0 | n24≠T0 | r T48 vs T0 | Equation used for estimation | np≠T0 | n48≠T0 | |||
| HCT | l/l | EDTA | 0.98 | HCT0 = HCT24 . 0.88 – 0.0039 | 0/44 | 11/44 | 0.97 | HCT0 = HCT48 . 0.84 – 0.0114 | 0/44 | 15/44 |
| EDCT | 0.99 | HCT0 = HCT24 . 1.03 – 0.0129 | 0/42 | 1/42 | 0.97 | HCT0 = HCT48 . 1.05 – 0.0296 | 0/42 | 1/42 | ||
| MCV | fl | EDTA | 0.96 | MCV0 = MCV24 . 0.89 – 0.51 | 0/44 | 10/44 | 0.85 | MCV0 = MCV48 . 0.86 – 2.10 | 1/44 | 17/44 |
| EDCT | 0.98 | MCV0 = MCV24 . 0.98 + 0.60 | 0/42 | 0/42 | 0.91 | MCV0 = MCV48 . 0.94 + 1.99 | 4/42 | 4/42 | ||
| MCHC | g/l | EDTA | 0.89 | MCHC0 = MCHC24 . 1.10 + 14.31 | 1/44 | 9/44 | 0.68 | MCHC0 = MCHC48 . 1.31 – 21.33 | 1/44 | 25/44 |
| EDCT | 0.95 | MCHC0 = MCHC24 . 0.81 + 59.24 | 1/42 | 0/42 | 0.84 | MCHC0 = MCHC48 . 0.73 + 84.15 | 3/42 | 1/42 | ||
HCT = haematocrit; MCHC = mean corpuscular haemoglobin concentration; MCV = mean corpuscular volume
Reticulocytes
In EDTA specimens, the RETc and RET% increased markedly: +39.2 % and +32.1%, respectively, at T24, and +70.9 % and +50.9 %, respectively, at T48. Moreover, the differences in the RETc exceeded the analytical variability in 36/43 cases and 39/43 cases at T24 and T48, respectively. On the scattergrams the reticulocyte dot plots showed a marked shift to the right in 35/44 cases. No statistical differences were observed for the reticulocyte maturation indexes (LFR, MFR, HFR) and the IRF, even though the differences exceeded the analytical variability in 0 to 19/43 cases.
In EDCT specimens, the RETc and RET% also increased progressively during storage, but a little more moderately for the RETc: + 23.3 % and +14.6 % at T24, respectively, and +41.5 % and +52.1 % at T48. The differences measured for the RETc exceeded the analytical variability: 29/42 and 40/42 at T24 and T48. The reticulocyte dot plot on the scattergrams showed a less pronounced right shift than with EDTA (22/42 cases). Finally, no statistical difference was observed for the reticulocyte maturation indexes (LFR, MFR, HFR) and the immature reticulocyte fraction (IRF), even though differences exceeded the analytical variability in 0 to 14/42 cases.
WBC
In EDTA, the leukocyte count remained stable for 48 h, few specimens showed differences exceeding the analytical variability and no specimen had been misclassified according to the RI. No statistical difference was observed for the neutrophil and lymphocyte counts during the storage period, even though the differences exceeded the analytical variability in 17/38 and 20/38 cases at T24 and T48, respectively, for the neutrophil count, and 27/38 and 17/38 cases at T24 and T48, respectively, for the lymphocyte count. Only 0 to 3/38 specimens were misclassified according to the RI. The monocyte count decreased by half at T48, 25/42 specimens showed variations that exceeded the analytical variation and only 1/42 specimens was misclassified according to the RI at T48. The eosinophil count increased gradually and slightly from T24, the differences exceeded the analytical variability in 19 and 26/ 41 cases at T24 and T48, respectively, and 2/41 specimens were classified differently according to the RI at T24 and T48. On the scattergrams the neutrophils dot plot clearly showed a right shift (42/44 cases), sometimes combined with dissociation into two populations in 14/44 cases. This caused the neutrophil and eosinophil dot plots to merge in 9/44 cases. The lymphocyte dot plot showed a down shift (19/44 cases), like the monocyte dot plot, which fell into the lymphocyte population (40/44 cases). The eosinophil dot plot showed a down shift (30/44 cases) and dispersion (36/44 cases).
In EDCT specimens, the WBC remained stable for 48 h of storage. The differential count showed fewer, but different, modifications than in EDTA. No statistical difference was observed for the lymphocyte and eosinophil counts, although the variation in lymphocyte count exceeded the analytical variation in 12 and 13/39 specimens at T24 and T48, respectively, and the variation in eosinophil count exceeded the analytical variation in 11 and 12/40 specimens at T24 and T48, respectively. Few specimens were classified differently according to the RI: 3 and 4/39 specimens for lymphocyte count at T24 and T48, respectively, and 3/40 specimens for eosinophil count at T24 or T48, respectively. The monocyte count decreased from T48, and neutrophil count increased slightly from T24 and remained stable between T24 and T48. The differences exceeded the analytical variation in 17/38 specimens for neutrophil count and in 15/41 specimens for monocyte count at T48. Only 2/38 specimens were classified differently according to the RI for neutrophil count at T48 and none for monocyte count. Dot plot changes were similar but less pronounced in EDCT than in EDTA.
PLT
No or very few statistical differences were observed for platelet variables when all the EDTA specimens were considered, whereas some differences could be observed when the specimens were divided into two groups according to the score of platelet aggregation (ie, HA and NLA). All EDCT results were analysed together because only 1/46 specimens had a high aggregation score (SPA-F). All the results are summarised in Table 2. On the scattergrams the optical platelet dot plot showed a down shift in 22/44 cases in EDTA and 29/42 cases in EDCT, a right shift in 22/44 cases in EDTA and 30/42 cases in EDCT, and an occasional dispersion in 29/44 cases in EDTA and 10/42 cases in EDCT.
In EDTA, the score of platelet aggregation (SPA-F) decreased at T24 in HA specimens, slightly increased in NLA specimens, and remained stable between T24 and T48 whatever the group. In the HA subgroup, the platelet count increased significantly with time, whatever the counting method (PLT-I, PLT-O or PLT-F). Differences obtained for PLT-I and PLT-O exceeded the analytical variability for almost all specimens (10 or 9/10 cases), and almost half the cases (4 or 5/10 cases) were classified differently according to the RI. In NLA specimens variation was irregular, differing with the method, and no time-related trend was observed. In these specimens differences exceeded the analytical variability in 24 and 20/33 cases and 12 and 14/33 cases at T24 and T48 for PLT-I and PLT-O, respectively. In addition, 1 or 0/33 cases and 0 /33 cases at T24 and T48 for PLT-I and PLT-O, respectively, were classified differently according to RI. No significant difference with time was observed for PLT-F in NLA specimens.
In EDCT specimens, SPA-F increased very slightly at T24, and then remained stable. The PLT-I and PLT-O counts decreased moderately and slightly from T24 respectively. Differences exceeding the analytical variation were observed in 39 and 38/41 cases and 17 and 10/42 cases at T24 and T48 for PLT-I and PLT-O, respectively. Few specimens were classified differently according to the RI: 7 and 3/41 cases and 2/42 cases at T24 and T48 for PLT-I and PLT-O, respectively. Finally, the PLT-F count showed a slight increase from T24 and then remained stable.
Discussion
To our knowledge, this is the first report about the stability of haematological variables in a large number of blood specimens collected from one healthy and 45 diseased cats into two different anticoagulants, EDTA and EDTA plus CTAD (EDCT), and analysed with modern equipment. Little information is available about changes occurring in haematological variables in EDTA feline blood specimens.1–5 Moreover, these studies were performed under different pre-analytical (different temperature and length of storage) and analytical (different haematological analyser) conditions, mostly with a small number of specimens, which preclude valid comparisons. In this study, because of the limited volume of the blood samples, it was decided to test two different anticoagulants and the effect of storage only at room temperature so as to more closely replicate conditions when a blood specimen has to be shipped to a reference laboratory.
RBC
As in previous reports, RBC, HGB and MCH1,3,5 did not change, or only changed very slightly, during storage at room temperature in the EDTA or EDCT specimens in our study. Haemoglobin has even been reported to remain stable for 96 h at room temperature, 4°C or after shipment. 1 The RBC count was also reported to increase in one case, 4 whereas it remained stable in other studies. As expected, HCT, MCV and MCHC changed markedly in EDTA specimens because of cell swelling.1,3,5 The increase of HCT and MCV has been reported to be more pronounced in specimens stored at room temperature than at 4°C.3,5 It was interesting to observe that no significant change occurred in EDCT specimens after 24 h and that the maximum change after storage for 48 h was below 5%, thus clinically irrelevant (Table 1). Furthermore, the more moderate increase of MCV in EDCT than in EDTA caused a smaller shift to the top of the RBC dot plots on the scattergrams (Figures 1 and 2). The RDWs also exhibited an increase, showing that RBC swelling was probably heterogeneous. The RDW had been reported to be almost stable for 3 days at 4°C and to be increased at 25°C. 3 Finally, time-related changes of HCT, MCV and MCHC in EDTA specimens due to cell swelling caused a relatively high rate of clinical misclassifications (between one third and one half of the cases) (Table 1). The latter can be reduced or fully compensated if the values at T0 are calculated according to the proposed equations (Table 3).
Reticulocytes
Reticulocyte percentage and count in EDTA and EDCT specimens increased similarly with storage. This is in agreement with previous results reported for EDTA feline blood with the same analyser.4,5 The order of magnitude of the RETc increase in EDTA was the same at T24 (about +40%) as in a study performed under the same conditions, 4 but was higher at T48 (+71%) than in another study performed at room temperature (+25%) and 4°C (+10%) with storage for 2 days. 5 The better stability in the latter study may be owing to the fact it was performed only with healthy cats. The cause of the reticulocyte increase needs to be investigated because there is not yet any explanation. In fact, owing to the maturation of reticulocytes with time, their count should be expected to decrease. Besides, no statistical difference was observed for the reticulocyte maturation indices after 48 h of storage at room temperature (Table 1), but no information is available about the clinical interpretation of these indices in feline blood. Possible clinical misclassifications could not be investigated for reticulocytes because RETc has been reported to notably differ according to the technology used.5,11,12 Finally, these RETc must be interpreted with caution as they were obtained in cats with mostly low or moderate RETc, and only in one cat with a high count. It may, therefore, be necessary to confirm these changes by investigating a larger population of cats with regenerative anaemia.
WBC
The WBC count was stable for 48 h of storage as reported in earlier studies using cytometry,4,5 whereas it had been reported to decrease or remain stable by impedance measurements.1–3 The differential counts are difficult to compare with previous results as the analytical conditions were different. In our study the differential count was quite stable. Neutrophil and lymphocyte counts were mostly stable or little changed for neutrophil count in EDCT, whereas the neutrophil count had been reported to increase and the lymphocyte count to decrease after 24 h of storage under the same conditions.4,5 The decrease of monocyte count and the increase of eosinophil count observed in our study are similar to previous observations.4,5 These changes in the counts may result from the collapse of the monocyte dot plot into the neutrophil dot plot and the right shift of the neutrophil dot plot towards the eosinophil dot plot. Modifications of the dot plots in EDCT were less pronounced than in EDTA specimens. Accordingly, the variation in the respective counts was more moderate in EDCT. The cascade of shifts observed on the scattergrams from monocyte to lymphocyte, lymphocyte to neutrophil and neutrophil to eosinophil dot plots could explain why the only reported changes were a decrease of monocyte count and an increase of eosinophil count, with a system of balance accounting for the minor changes in neutrophil and lymphocyte counts.
PLT
The stability of platelet count with storage has been very little studied. Results obtained with the Sysmex XT-2000iV analyser were contradictory,4,5 but platelet aggregation was not investigated. This is the first study to investigate the evolution of platelet aggregation with storage of feline specimens. Platelet clumping and large platelets are frequent in cats, making it difficult to obtain reliable counts in EDTA specimens. Changes in the aggregation score depended on the initial value (at T0), decreased dramatically in HA EDTA specimens, and increased slightly in EDTA and EDCT specimens showing no or low aggregation (NLA). Accordingly, platelet counts depended on the degree of aggregation. They were also markedly increased between T0 and T24, and more moderately between T24 and T48 in HA EDTA specimens whatever the method of counting, as the degree of aggregation decreased progressively and markedly during storage. Platelet counts in NLA EDTA specimens and in EDCT specimens showed no or only slight changes over time. A tendency to decrease was observed for PLT-I and more moderately for PLT-O, which could be explained by the very moderate increase in the degree of aggregation and/or by the increasing size of the platelet with storage.
Conclusions
Delayed analysis of feline blood specimens stored at room temperature and measured by the Sysmex XT-2000iV analyser leads to a significant increase in MCV, HCT, reticulocyte and eosinophil counts, and a significant decrease in MCHC and monocyte count, whereas HGB, RBC, MCH, WBC, and neutrophil and lymphocyte counts remain stable. Moreover, the changes are generally more pronounced in EDTA than in EDCT. Changes in the platelet counts of a specimen followed the evolution of the aggregation score which decreased markedly in initially HA specimens, and increased slightly in NLA specimens. Finally, CTAD can reduce the changes in the different haematological variables observed in feline blood samples during storage, and thus improves the reliability of a CBC and limits clinical misinterpretations.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The Sysmex XT-2000iV analyser used in this study was made available as a free loan by the manufacturer, who was not involved in any step or funding of this study.
Accepted: 6 November 2012
References
- 1. Fontaine M, Hamelin N, Paradis M. Effect of time, storage conditions and mailing on the stability of feline blood parameters. Med Vet Quebec 1986; 16: 157–164. [Google Scholar]
- 2. Grenn HH, Atkinson SM, Carlos B, Sobol B. Investigational studies of selected hematological parameters in fresh and mailed blood of six species of domestic animals. Can Vet J 1976; 17: 213–215. [PMC free article] [PubMed] [Google Scholar]
- 3. Pastor J, Cuenca R, Velarde R, et al. Evaluation of a hematology analyzer with canine and feline blood. Vet Clin Pathol 1997; 26: 138–147. [DOI] [PubMed] [Google Scholar]
- 4. Weissenbacher S, Riond B, Hofmann-Lehmann R, Lutz H. Evaluation of a novel haematology analyser for use with feline blood. Vet J 2011; 187: 381–387. [DOI] [PubMed] [Google Scholar]
- 5. Bauer N, Nakagawa J, Dunker C, et al. Evaluation of the automated hematology analyzer Sysmex XT-2000iV compared to the ADVIA (R) 2120 for its use in dogs, cats, and horses. Part II: Accuracy of leukocyte differential and reticulocyte count, impact of anticoagulant and sample aging. J Vet Diagn Invest 2012; 24: 74–89. [DOI] [PubMed] [Google Scholar]
- 6. Granat F, Geffré A, Braun JP, Trumel C. Comparison of platelet clumping and complete blood count results with Sysmex XT-2000iV in feline blood sampled on EDTA or EDTA plus CTAD (citrate, theophylline, adenosine, and dipyridamole). J Feline Med Surg 2011; 13: 953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Georges JW. Ocular field width and platelet estimates. Vet Clin Pathol 1999; 28: 126. [Google Scholar]
- 8. Norman EJ, Barron RC, Nash AS, Clampitt RB. Evaluation of a citrate-based anticoagulant with platelet inhibitory activity for feline blood cell counts. Vet Clin Pathol 2001; 30: 124–132. [DOI] [PubMed] [Google Scholar]
- 9. Lilliehook I, Tvedten H. Validation of the Sysmex XT-2000iV hematology system for dogs, cats, and horses. II. Differential leukocyte counts. Vet Clin Pathol 2009; 38: 175–182. [DOI] [PubMed] [Google Scholar]
- 10. Bourgès-Abella N, Geffré A, Concordet D, et al. Canine hematology reference intervals for the XT-2000iV analyzer. Vet Clin Pathol 2011; 40: 303–315. [DOI] [PubMed] [Google Scholar]
- 11. Moritz A, Fickenscher Y, Meyer K, et al. Canine and feline hematology reference values for the ADVIA 120 hematology system. Vet Clin Pathol 2004; 33: 32–38. [DOI] [PubMed] [Google Scholar]
- 12. Lilliehook I, Tvedten H. Validation of the Sysmex XT-2000iV hematology system for dogs, cats, and horses. I. Erythrocytes, platelets, and total leukocyte counts. Vet Clin Pathol 2009; 38: 163–174. [DOI] [PubMed] [Google Scholar]