Abstract
This study compared indirect blood pressure measurements using a non-invasive method, high-definition oscillometry (HDO), with direct measurements using a radio-telemetry device in awake cats. Paired measurements partitioned to five sub-ranges were collected in six cats using both methods. The results were analysed for assessment of correlation and agreement between the two methods, taking into account all pressure ranges, and with data separated in three sub-groups, low, normal and high ranges of systolic (SBP) and diastolic (DBP) blood pressure. SBP data displayed a mean correlation coefficient of 0.92 ± 0.02 that was reduced for low SBP. The agreement level evaluated from the whole data set was high and slightly reduced for low SBP values. The mean correlation coefficient of DBP was lower than for SBP (ie, 0.81 ± 0.02). The bias for DBP between the two methods was 22.3 ± 1.6 mmHg, suggesting that HDO produced lower values than telemetry. These results suggest that HDO met the validation criteria defined by the American College of Veterinary Internal Medicine consensus panel and provided a faithful measurement of SBP in conscious cats. For DBP, results suggest that HDO tended to underestimate DBP. This finding is clearly inconsistent with the good agreement reported in dogs, but is similar to outcomes achieved in marmosets and cynomolgus monkeys, suggesting that this is not related to HDO but is species related. The data support that the HDO is the first and only validated non-invasive blood pressure device and, as such, it is the only non-invasive reference technique that should be used in future validation studies.
Introduction
In middle-aged and older cats, arterial blood pressure (BP) is a key parameter to assess for identification of systemic hypertension associated with target organ damage and even more before the occurrence of any damage. The measurement of BP is, in addition, important to evaluate the efficacy of antihypertensive therapy. 1 Various methods for BP measurements are applicable in clinical patients. In anaesthetised animals, BP can be measured directly by means of an intra-arterial catheter connected to a pressure sensor, and this approach is considered the gold standard method. Direct measurement of BP has been applied to conscious animals, 2 but is technically difficult and not routinely available. A second approach utilises indirect non-invasive methods, which are generally more clinically applicable. 3 The two most commonly used non-invasive methods, Doppler sphygmomanometry and oscillometry, use a cuff placed on a limb or the tail. These indirect methods provide estimates of the systolic BP (SBP), but none have met validation criteria as defined by the American Association of Medical Instrumentation (AAMI) 4 or modified criteria suggested by an American College of Veterinary Internal Medicine (ACVIM) consensus panel 3 for validation of automated non-invasive blood pressure devices. More recently, a new method of indirect BP measurement, high-definition oscillometry (HDO), has been developed. As with other indirect methods, the BP is measured at the level of a peripheral artery with a cuff placed on a forelimb or the tail, and these methods provide SBP, diastolic BP (DBP) and mean BP (MBP), which are obtained from the analysis of the waveforms of recorded pressure oscillations. In cats, only one evaluation study of HDO has been published at present. 5 This evaluation was performed by comparison of measurements obtained by a Doppler ultrasonic device with direct BP measurements undertaken in anaesthetised animals. The authors concluded that discrepancies between the techniques were achieved. However, in this study, many of the comparisons were made in anaesthetised animals and are thus of questionable applicability to conscious cats. In conscious cats 6 and dogs, 7 comparisons of an indirect oscillometric device and a Doppler ultrasonographical device with direct BP measurements have shown that neither met the criteria for validation of AAMI 4 or the ACVIM consensus panel. 3 The purpose of this study was to compare SBP and DBP values obtained by HDO measurements with values achieved simultaneously using a well-recognised direct method,6–13 using radio-telemetry, in conscious healthy cats. In addition, to reproduce the recording conditions expected in hypertensive, normotensive and hypotensive animals, this evaluation was performed at ranges of BP corresponding to low, normal and high BP levels.
Material and methods
Animals
All animals used in this study were handled and cared for in accordance with the Directive 86/609/EEC European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. 14 The study protocol related to this experiment was reviewed and approved by the internal ethics committee.
Purpose-bred European cats (four males and two females, aged 6–8 months old and weighing 2.4–3.7 kg) were used in this study. During the acclimatisation period of 2 weeks, and apart from the recording sessions, animals of the same sex were group-housed. Toys (balls, scraper or other), as well as different floors, were used for enrichment. The animals were placed in a temperature-regulated (15–21°C) animal house kept at a relative humidity between 45% and 65% (except during the cleaning slot) with non-recycled filtered air changed approximately 10 times per hour. Tap water was provided at libitum. Likewise, food (Special Diets Services (SDS), reference: Diet F for cat) was available ad libitum.
Telemetry device description and implantation
The telemetry device and the method used for instrumentation of cats were derived from the method described previously by Miller et al. 9 The telemetry device (model TL11M2D70PCT; Data Sciences International, St Paul, MN, USA) includes a hermetically-sealed transmitter body, containing an electronics module, sensor and battery that is attached to a fluid-filled catheter. After instrumentation, the battery can be turned on and off through the skin with a magnet. The catheter consists of polyethylene tubing coated with anti-thrombogenic film and a thin-walled, gel-filled tip. The gel prevents blood diffusion and conveys pressure changes to the sensor in the transmitter body.
During the instrumentation, all procedures were conducted according to aseptic surgical methods. Anaesthesia was induced using propofol (Rapinovet) administered at 8 mg/kg (IV) and was maintained by a continuous intravenous (IV) infusion at 0.04–0.06 ml/kg/min throughout the surgery. Buprenorphine (Temgesic) at 0.01 mg/kg was administered intramuscularly before the start of the surgery to avoid surgical pain. Radio-telemetry transmitters were implanted subcutaneously. The sensor of the transmitter was introduced into a femoral artery up to the abdominal aorta. Postsurgical analgesia was provided by administration of meloxicam 0.07 mg/kg (Metacam) given orally, once a day for 2–5 days after the surgery. Prophylactic antibiotic therapy, 30 mg/kg amoxicillin (SC), was administered daily for 1 week after surgery. At least 2 weeks of recovery were allowed after the surgery before starting the experiment to ensure BP reached a steady state, as observed after cat instrumentation with the telemetry device. 9
Experimental design
The BP was measured in each cat to collect at least 25 SBP and DBP readings per animal and per range, and for the five sub-ranges respectively (SBP: ≤109, 110–129, 130–149, 150–169, ≥170 mmHg; DBP: ≤69, 70–89, 90–109, 110–129, ≥130 mmHg). According to this study design, a total of at least 750 SBP/DBP readings were generated. Two vasoactive agents, amlodipine (1 mg/kg, PO) and phenylephrine (0.1, 0.3, 1, 3 and 10 mg/kg, PO) were administered in order to reach the lower and the upper parts of the SBP/DBP ranges defined above. Amlodipine was chosen because this calcium channel blocker induces a stable and long lasting hypotension related to peripheral vasodilatation with only minor reflex tachycardia. Phenylephrine produces a peripheral vasoconstriction resulting in relatively stable hypertension after oral administration. The oral route was chosen because a comparatively stable, long-lasting effect was achieved when compared with other routes of administration, such as the IV route. Moreover, the oral route was less painful than the IV and thus more appropriate for repeated administration in the same animal. Medications, amlodipine or phenylephrine, were administered approximately 0.5–1 h before measurements of BP. Only one vasoactive agent was administered within a day. For phenylephrine specifically, two ascending dosage levels at most were tested per day for the same animal. A maximum of five sessions of measurements (ie, 5 sets × 5 readings/set) was performed for each animal per day. No randomisation of the order of vasoactive agent administration was made as the objective was to collect 25 SBP or DBP paired measurements (ie, HDO/telemetry) for each animal divided in the five sub-ranges of blood pressure (ie, five values/animal/range).
BP measurement
The measurements were performed in a quiet laboratory room close to the animal room. Telemetric measurements of BP were recorded using a RMC-1 biotelemetry receiver placed close to the animal. Simultaneously, SBP and DBP were measured at the level of the tail artery using the HDO detector (High Definition Oscillometry device; S + B MedVET). A set of five successive, simultaneous readings was performed. The measurements were performed under quiet conditions and over the shortest possible period (ie, within 3 mins). Telemetric measurements of BP (figures and waveforms) were recorded continuously at a sampling rate of 500 Hz for the entire duration of the HDO measurements. The minimum and maximum SBP and DBP values were determined from the telemetry system using the continuous telemetry waveform trace recorded during the HDO measurement. The HDO value was then compared with the telemetry SBP/DBP range, and the delta was the smallest difference. When the HDO value fell in the telemetry range, the delta was 0 mmHg. If the HDO value was below the telemetry range, the delta was the minimum blood pressure value from the telemetry system minus the HDO value. If the HDO value is higher than the telemetry range, the delta was the HDO value minus the maximum blood pressure value from telemetry system. Heart rate was calculated from arterial blood pressure waveforms achieved by telemetry.
Statistical analysis
At least 25 paired readings were collected per animal, both by HDO and telemetry methods (ie, five paired HDO/telemetry values/animal/BP range). The results were analysed separately for each animal taking into account all pressure ranges together, then for low (<110 mmHg), normal (110–149 mmHg) and high (>150 mmHg) ranges of SBP, and for low (<90 mmHg), normal (90–120 mmHg) and high (>120 mmHg) ranges of DBP.
Differences between paired readings of SBP and DBP obtained from the methods were calculated. The agreement level between the two recording devices was assessed in each pressure range using the method described by Bland–Altman. 15 The differences between values achieved with the two methods were plotted against the mean of all paired measurements. The bias between the two methods was assessed from the mean difference of the two methods. A positive or negative bias would reflect an under- or overestimation of BP values by the HDO method in relation to the reference method (ie, telemetry). The agreement between the two methods was assessed from the mean difference ± 2 SD. The percentage of paired measurements lying within a difference of less than 10 mmHg and less than 20 mmHg for the two methods was calculated as recommended by the ACVIM consensus panel. 3 Finally, linear regression curves were built for calculations of correlation coefficient (r) between the two methods. Calculations, graphs and correlation analysis were processed using RS/1 software (release 6.0.1; APPLIED MATERIALS).
Drugs
Amlodipine besylate and phenylephrine hydrochloride were purchased from Sigma-Aldrich. The vehicle for both molecules was sterile water. Formulations were prepared daily and kept for a maximum of 8 h, at room temperature, away from light. Amlodipine and phenylephrine were administered orally in a volume of 1 ml/kg.
Results
When comparing SBP values collected using the HDO and telemetry methods, the correlation coefficients (CC) of the paired measurements between the two methods were between 0.91 and 0.98 for all but one cat, whose CC was 0.85, resulting in an overall mean CC for the six cats of 0.92 ± 0.02. In this latter animal, the CC was reduced owing to differences between HDO and telemetry values within the low range of SBP (ie, <110 mmHg) in this cat. When SBP data were partitioned as low (n = 5–6 measurements/cat), normal (n = 14–19 measurements/cat) and high (n = 10–13 measurements/cat) pressure level groups, CCs were 0.71 ± 0.26, 0.78 ± 0.09 and 0.87 ± 0.07, respectively, confirming that the correlation between the two methods was increased for higher SBP levels (Table 1; Figure 1).
Table 1.
Correlation between systolic blood pressure (SBP) and diastolic blood pressure (DBP) values measured with high-definition oscillometry and telemetry
| Parameter | Pressure group | Correlation coefficient |
|---|---|---|
| SBP | Low | 0.71 ± 0.26 n = 5–6 |
| Normal | 0.78 ± 0.09 n = 14–19 |
|
| High | 0.87 ± 0.07 n = 10–13 |
|
| Overall | 0.92 ± 0.02 n = 25 |
|
| DBP | Low | 0.51 ± 0.21 n = 7–19 |
| Normal | 0.40 ± 0.34 n = 6–20 |
|
| High | 0.51 ± 0.29 n = 5–9 |
|
| Overall | 0.81 ± 0.02 n = 25 |
n = number of measurements used for individual correlation analysis for each cat
Mean (± SD) data calculated from individual data obtained in six animals
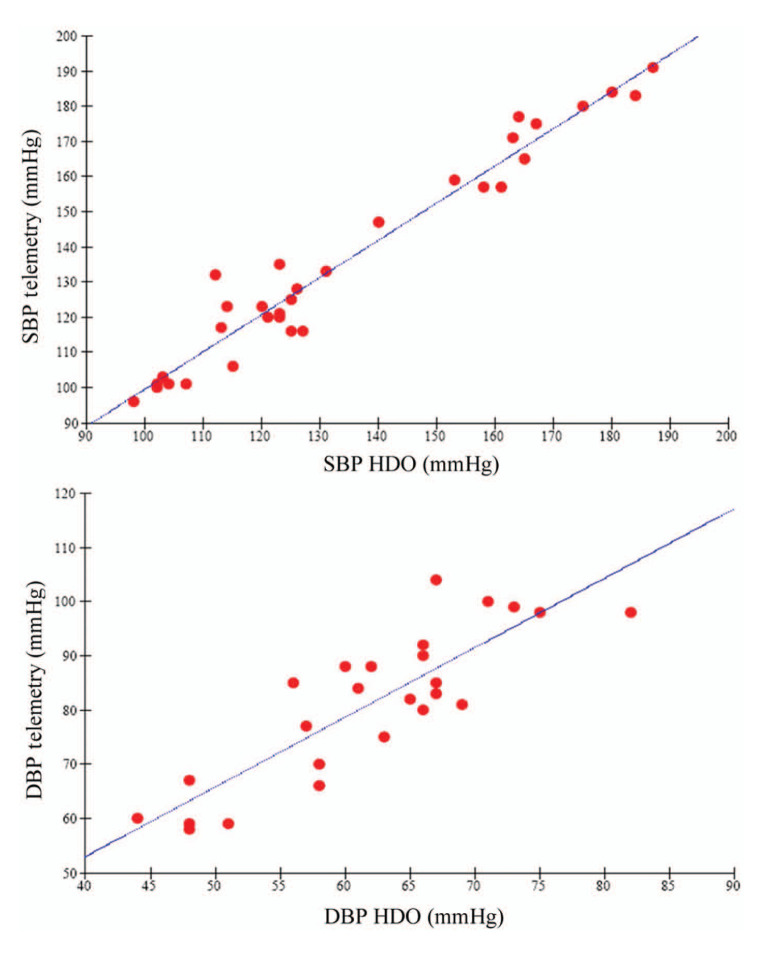
Figure 1.
Linear regression between systolic blood pressure (SBP) and diastolic blood pressure values measured with high-definition oscillometry (HDO) and telemetry in an individual animal, cat number 2008024. SBP correlation coefficient r = 0.98; DBP correlation coefficient r = 0.86
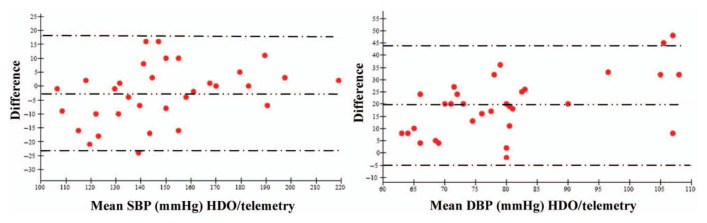
The relevance of HDO for a faithful measurement of SBP over a large range of pressure levels (Figure 2) was high, as supported by the low mean bias achieved (ie, –2.2 ± 1.1 mmHg). Likewise, when SBP values were separated in the different sub-groups (Figure 3), the bias remained very limited, but it was clearly impaired for low SBP values (ie, –10.8 ± 11.3 mmHg), suggesting HDO slightly overestimated SBP compared with telemetry during hypotensive conditions. Nevertheless, in the low SBP subgroup, the 95% limit of agreement was the narrowest and all paired values were within 10 mmHg (Table 2), supporting a very good correspondence between the two methods.
Figure 2.
Bland–Altman plot of agreement between systolic blood pressure (SBP) and diastolic blood pressure (DBP) measured with high-definition oscillometry and telemetry in cat number 2008018. Mean difference (bias): – -- – -- – -- –, low limit of agreement (–2 SD) and high limit of agreement (+ 2SD): – - – - – - –. SBP: bias = −2.7 mmHg; limits of agreement = ± 20.7 mmHg; percentage of paired measurements lying within a difference between the two methods less than 10 mmHg = 84%; less than 20 mmHg: 100%. DBP: bias = 19.5 mmHg; limits of agreement = ± 24.5 mmHg; percentage of paired measurements lying within a difference between the two methods less than 10 mmHg = 25%; less than 20 mmHg = 47%
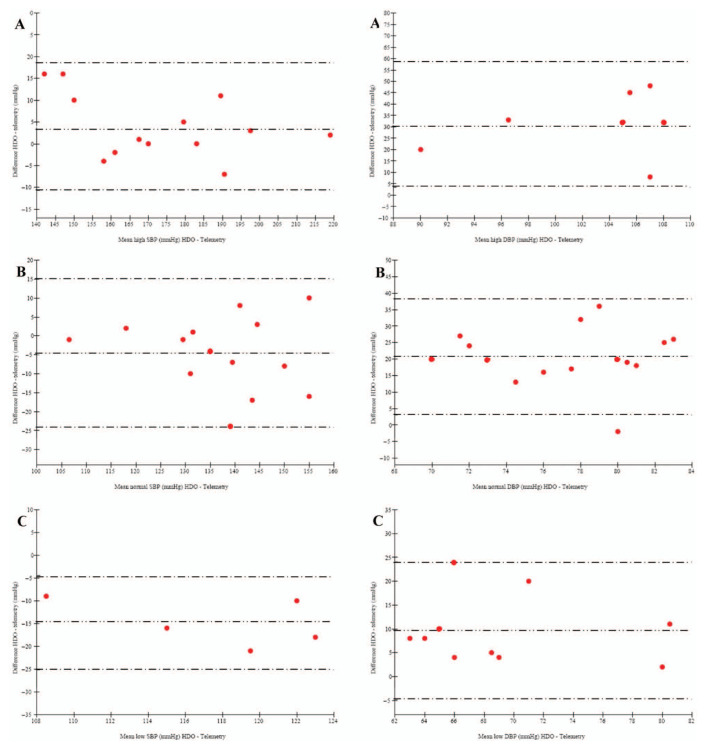
Figure 3.
Bland–Altman plot of agreement between systolic blood pressure (SBP) and diastolic blood pressure (DBP) measured with high-definition oscillometry (HDO) and telemetry in cat number 2008018 according to subgroups (A = high; B = normal; C = low). Mean difference (bias): – -- – -- – -- –, low limit of agreement (–2 SD) and high limit of agreement (+ 2 SD): – - – - – - –. SBP high, normal, low: bias: 3.9, –4.6, –14.8 mmHg; limits of agreement: ± 14.6, ± 19.5, ± 10.3 mmHg; percentage of paired measurements lying within a difference between the two methods less than 10 mmHg: 69, 93, 100%; <20 mmHg: 100, 100, 100%. DBP high, normal, low: bias: 31.1, 20.7, 9.6 mmHg; limits of agreement: ± 27.6, ± 17.6, 14.4 mmHg; percentage of paired measurements lying within a difference between the two methods less than 10 mmHg: 14, 7, 100%; <20 mmHg: 14, 40, 80%
Table 2.
Agreement between systolic blood pressure (SBP) and diastolic blood pressure (DBP) values measured with high-definition oscillometry and telemetry
| Parameter | Pressure group | Bias (mmHg) | Limits of agreement (mmHg) | % of paired measurement within ± 10 mmHg | % of paired measurement within ± 20 mmHg |
|---|---|---|---|---|---|
| SBP | Low | −10.8 ± 11.3 | 12.1 ± 4.1 | 100 ± 0 | 100 ± 0 |
| Normal | −3.4 ± 3.8 | 17.6 ± 1.8 | 94 ± 6 | 98 ± 3 | |
| High | 2.9 ± 1.3 | 20.6 ± 8.0 | 77 ± 13 | 92 ± 9 | |
| Overall | –2.2 ± 1.1 | 21.6 ± 2.6 | 88 ± 3 | 96 ± 2 | |
| DBP | Low | 14.6 ± 5.5 | 14.3 ± 4.9 | 27 ± 25 | 67 ± 29 |
| Normal | 24.6 ± 2.0 | 15.7 ± 4.1 | 3 ± 4 | 20 ± 15 | |
| High | 36.0 ± 6.3 | 22.3 ± 9.4 | 7 ± 10 | 13 ± 13 | |
| Overall | 22.3 ± 1.6 | 22.6 ± 1.9 | 13 ± 4 | 38 ± 7 |
Mean (± SD) data calculated from individual data achieved in six animals
Overall, the percentages of paired SBP measurements lying within a difference of less than 10 and 20 mmHg between the two methods was 88 ± 3% and 96 ± 2%, respectively. A higher discrepancy was found at the high SBP levels, specifically for the 10 mmHg difference.
When comparing DBP individual values collected using the HDO and the telemetry methods, the CCs of the paired measurements between the two methods were between 0.73 and 0.86 individually, with a mean CC of 0.81 ± 0.02, suggesting a lower correlation between the two methods for DBP measurement compared with SBP. When evaluated on data in three subgroups of pressure level, the CC was dramatically reduced owing, most likely, to the limited number of observations and limited distribution of values within each pressure range (Table 1). The bias between the two methods was 22.3 ± 1.6 mmHg, indicating that HDO measurement of DBP tended to produce lower values than the telemetry devices. Likewise, the limit of agreement between the two methods for DBP measurement was 22.6 ± 1.9 mmHg. The percentages of paired measurements lying within 10 or 20 mmHg, were 3–27% and 16–56%, respectively, confirming the discrepancy between the two methods for DBP measurement. When evaluating the agreement level between the methods for DBP values in the three pressure ranges, the bias was dependent on the pressure range with the smaller bias observed at low DPB.
Running additional pulse wave analyses to identify correlation of SBP and DBP, a high correlation was found for SBP, whereas a similar poor correlation was observed for DBP. However, these analyses suggested a good correlation between DBP measured with telemetry and MBP measured with HDO. This observation was confirmed by correlation analyses performed on these data showing a CC of 0.90 ± 0.02. In addition, a good correspondence between DBP by HDO and MBP by telemetry was achieved when tested by means of the Bland–Altman method (Table 3).
Table 3.
Agreement between diastolic blood pressure (DBP) values measured with telemetry (Tel) and mean blood pressure (MBP) measured with high-definition oscillometry (HDO)
| Parameter | Bias (mmHg) | Limits of agreement (mmHg) | % of paired measurement within ± 10 mmHg | % of paired measurement within ± 20 mmHg |
|---|---|---|---|---|
| DBP Tel/MBP HDO | 1.7 ± 0.7 | 14.9 ± 2.3 | 93 ± 5 | 99 ± 2 |
Mean (± SD) data calculated from individual data achieved in six animals
We assessed heart rate during the simultaneous BP measurements with HDO measurement through the experiment. On the first day of BP measurement with HDO, the mean level of heart rate during the recording was 197 ± 6 beats/min. On the following days, heart rate during measurement decreased to achieve a level that remained below 180 beats/min, suggesting that animals were accustomed to the recording conditions and were apparently undisturbed by the HDO measurement (Figure 4).
Figure 4.
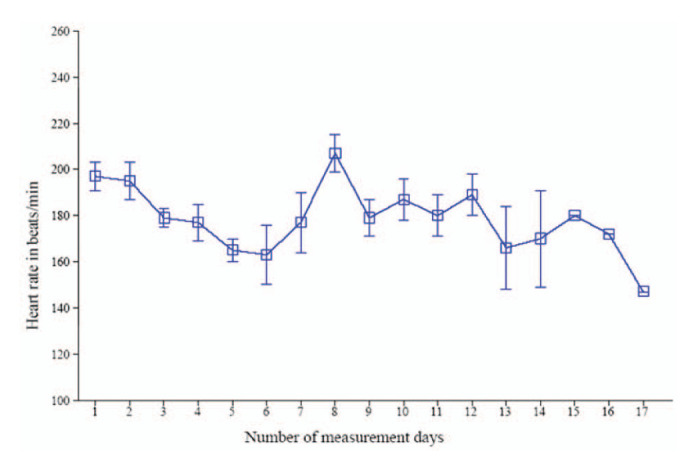
Mean (± SD) heart rate level in cats during high-definition oscillometry measurement over the course of the overall experiment
Discussion
Hypertension in cats is mainly secondary to an underlying pathology, particularly in cats with chronic kidney disease. To predict outcomes in affected cats, proteinuria is arguably a better clinical parameter than measurement of BP. 16 However, BP measurements in studies of spontaneous chronic kidney disease in cats have relied upon devices that meet neither the AAMI nor the ACVIM criteria for validation, 6 which may have compromised the reliability of BP measurements. Future studies will be necessary to assess the reliability of BP measurements obtained with a validated device.
It is now established that about 20% of hypertensive cats have idiopathic hypertension. 17 The diagnosis of idiopathic hypertension is based on elevated SBP and DBP above 150 mmHg and 95 mmHg, respectively, associated with target organ damage (TOD) depending on its risk category. 3 Hypertensive cats frequently display TOD, but when BP is slightly increased and thus associated with a low risk of TOD or in the acute phase of greater BP increases, TOD is absent. In this situation the diagnosis of hypertension requires reliable BP measurements.
A reliable measurement of BP in cats must overcome at least two potential sources of error. First, potential bias in data interpretation can occur in conscious subjects owing to the ‘white coat effect’, which is a pseudonym for artifactual BP increases related to anxiety and/or excitement caused by the measurement condition. To manage this risk of bias, the ACVIM consensus panel defined a standardised measurement process.
The second source of error is related to the method used for BP measurement in the clinical environment. While the methods recognised as the most accurate and reliable are direct invasive measurements using an intra-arterial catheter, 18 the invasiveness and technical difficulty of these methods has limited their clinical acceptance, and indirect BP measurement methods are widely preferred.
In this study, we used as a reference method implanted telemetry, which is a direct and invasive BP measurement method applicable for long-term measurement in non-sedated animals. This method was subjected to numerous validation studies in various species, including rats, 19 dogs, 20 monkeys21–23 and cats, 24 and which is recognised as a gold standard. Therefore, we compared HDO measurements under measurement conditions matching with the ACVIM consensus panel’s recommendation by comparing data achieved with the gold standard method for BP measurement in non-sedated animals. Moreover, in order to cover various clinical states, measurements were not focused on normal BP values only, but included high and low BP pressure levels similar to BP levels expected in case of hypertension or hypotension, respectively.
With this approach, HDO displayed a good agreement level, meeting the AAMI 3 requirement defined for BP measurement in humans, ie, 5 ± 8 mmHg of the measurements obtained with the reference method. However, less than 95% of the HDO data lay within 10 mmHg of the reference telemetry measurements, as required for AAMI validation. Nevertheless, if we consider the updated criteria defined by the ACVIM consensus panel, 2 which take into account specific challenges when measuring BP in conscious animals, these data indicate that HDO provides a faithful measurement of SBP in conscious cats. To our knowledge, this is the first study in which an indirect BP technique, here HDO, has met the validation criteria of the ACVIM consensus panel.
In healthy humans25,26 or rodents, 27 owing to the mechanical properties of the vasculature along the arterial tree, SBP is higher at peripheral sites, the brachial or tail artery compared with a central site such as the thoracic aorta. Direct SBP measurements in cats were performed in the distal abdominal aorta, whereas HDO measurements were performed at the level of the coccygeal artery. The absence of significant difference between the two methods in measuring SBP suggests that the measurement sites chosen were close enough to enable this comparison. When simultaneous SBP measurements were obtained at low BP levels, ie, <110 mmHg, HDO overestimated SBP by about 10 mmHg. The reduced number of observations available for analyses when data were allocated in SBP level sub-groups likely contributed to this finding, but these results are in agreement with findings achieved by Petric et al, 5 which were obtained in anaesthetised cats using the Doppler ultrasonic method as reference. They reported a SBP overestimation of 8.56 mmHg when SBP was spontaneously below 100 mmHg. 5 However, in our study, low levels of SBP were achieved by means of the administration of amlodipine, a calcium channel blocker known to reduce BP by inducing a vasodilatation. 28 In the presence of spontaneous hypotension, reflex tachycardia is common. As heart rate influences pulse wave velocity, tachycardia would be expected to affect SBP at levels upstream in the arterial tree. However, as we observed in previous studies (data not shown), the heart rate during BP measurements after pretreatment with amlodipine in cats indicate that reflex tachycardia is not induced. Amlodipine’s vasorelaxant action is not only due to the blockade of voltage-gated calcium channels but also to the release of nitric oxide from the vascular endothelium resulting in a pronounced reduction in the vascular resistance and stiffness. 29 Experimental data obtained in rats showed that vasoactive drugs, angiotensin-converting enzyme inhibitors and calcium channel blockers also affect the mechanical properties of large arteries, reducing central pulse pressure (PP) with practically no effect on the peripheral aorta PP. 27 The effect of the vascular mechanical properties on the SBP along the arterial tree, the differences in the BP measurement sites and the mechanism of action of amlodipine on the vasculature could explain why we observed a discrepancy between the two methods when measuring low SBP values specifically.
When transposed in the context of clinical use for the diagnosis of systemic hypertension, this feature of HDO seems devoid of major effect in terms of interpretation. Indeed, in a clinical situation SBP measurement in conscious cats is intended to identify systemic hypertension or to support the efficacy of an antihypertensive treatment. Ideally, for the latter use SBP might be expected to lie within the normal range, but not to be below the normal range. In both cases, our results suggest that HDO would provide reliable measurements.
For DBP, despite a good correlation, we observed a low level of agreement between these two methods, and HDO tended to underestimate the DBP. The measurement site in the arterial tree seems unlikely to be responsible for this finding, as DBP and MBP measurements are less dependent on the location in the arterial tree than SBP. 26 The accuracy of implanted telemetry pressure transducers is stable after instrumentation, although chronically implanted devices may undergo a slight baseline drift, which can affect both SBP and DBP. 30 In our study, the telemetry devices remained implanted for up to 3 months, but there was no change in baseline values, which were recorded prior to instrumentation and after the telemetry implant was removed, suggesting that the baseline remained stable without drift. The amplitude of the pulse pressure as measured by telemetry was typically 30–40 mmHg. When calculated from HDO measurements, the pulse pressure was above 50–70 mmHg. In people, normal pulse pressure measured at a peripheral site is 35–40 mmHg. In small mammals with greater heart rates, the pulse pressure is usually lower because of the lower stroke volume and differences in the vascular resistance. 25 Consequently, the high pulse pressures determined from HDO measurements are suspiciously high, suggesting that DBP measured with HDO was, indeed, underestimated.
Measurement of DBP in conscious cats is considered technically challenging, and this tends to be supported by the limited amount of data for DBP in the literature compared with that for SBP. In conscious healthy cats, HDO was found to provide higher DBP estimates than a Doppler ultrasonic device, while SBP was similar. 31 However, compared with direct telemetry, the Doppler ultrasonic method is clearly less robust and questionable as a reference method. 6 This limitation has been addressed by others in conscious cats using an oscillometric device. As for HDO in the present study, it has been reported that these older oscillometric devices yield an underestimation of about 20 mmHg for both SBP and DBP. 6
Nevertheless, in other species, such as the dog, a good agreement level was found between DBP measured by telemetry and HDO at the level of the tail artery. 32 The reason why DBP measurement with HDO is slightly less comparable in cats than in dogs remains unclear. In cynomolgus monkeys 23 and in marmosets 33 HDO readings achieved at the level of the tail artery tended to be lower than with telemetry. The common feature of this species with cats, compared with dogs, is a greater heart rate level, which could affect the condition of DBP estimation with HDO.
Conclusions
When compared with the gold standard method for BP measurement in conscious animals, HDO measurement of SBP met the validation criteria of the ACVIM consensus panel, supporting the sensitivity and the robustness of this method. For DBP, HDO tended to provide mild underestimations, which may be species related. The data support that the HDO detector is the first and only validated non-invasive blood pressure device, and, as such, HDO is the only non-invasive reference technique that should be used in future validation studies. However, it should be stressed that these results were obtained in only six cats, and therefore the findings need to be confirmed in a larger number of animals. In addition, the cats involved in the present study were accustomed in advance to the measurement methods and were handled regularly. This feature differs from standard clinical practice where patients undergo blood pressure measurement less frequently. Therefore, the influence of acclimatisation and handling should be checked in clinical patients.
Acknowledgments
We acknowledge the contribution of Karine Guedes for her experimental assistance.
Footnotes
Funding: This work was supported by Novartis Animal Health Inc, Werk Rosental, Basel, Switzerland.
The authors do not have any potential conflicts of interest to declare.
Accepted: 27 May 2013
References
- 1. Stepien RL. Feline systemic hypertension: diagnostic and management. J Feline Med Surg 2011; 13, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stepien RL, Rapoport GS, Henik RA, et al. Comparative diagnostic test characteristics of oscillometric and Doppler ultrasonographic methods in the detection of systolic hypertension in dogs. J Vet Intern Med 2003; 217: 65–72. [DOI] [PubMed] [Google Scholar]
- 3. Brown S, Atkins C, Bagley R, et al. ACVIM consensus statement, guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 4. ANSI/AAMI SP10:2002. American Association of Medical Instrumentation, Standards for the performance of automated non-invasive blood pressure devices. [Google Scholar]
- 5. Petric AD, Petra Z, Jerneja S, Alenka S. Comparison of high definition oscillometric and Doppler ultrasonic devices for measuring blood pressure in anaesthetized cats. J Feline Med Surg 2010; 12: 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haberman CE, Morgan JD, Kang CW, Brown SA. Evaluation of Doppler ultrasonic and oscillometric methods of indirect blood pressure measurement in cats. Intern J Appl Res Vet Med 2004; 2: 279–289. [Google Scholar]
- 7. Haberman C, Morgan J, Brown S. Evaluation of Doppler ultrasonic and oscillometric methods of indirect blood pressure estimation in conscious dogs. Can J Vet Res 2006; 70: 211–217. [PMC free article] [PubMed] [Google Scholar]
- 8. Kurtz TW, Griffin KA, Bidani AK, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 2: blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45: 299–310. [DOI] [PubMed] [Google Scholar]
- 9. Miller RH, Smeak DD, Lehmkuhl LB, et al. Radiotelemetry catheter implantation: surgical technique and results in cats. Contemp Top Lab Anim Sci 2000; 39: 34–39. [PubMed] [Google Scholar]
- 10. Mathur S, Brown C, Dietrich U, et al. Evaluation of a technique of inducing hypertensive renal insufficiency in cats. Am J Vet Res 2004; 65: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 11. Buranakarl C, Mathur S, Brown S. Effects of dietary sodium chloride intake on renal function and blood pressure in cats with normal and reduced renal function. Am J Vet Res 2004; 65: 620–627. [DOI] [PubMed] [Google Scholar]
- 12. Mathur S, Syme H, Brown C, et al. Brown S. Effects of the calcium channel antagonist amlodipine in cats with surgically induced hypertensive renal insufficiency. Am J Vet Res 2002; 63: 833–839. [DOI] [PubMed] [Google Scholar]
- 13. Belew A, Brown SA. Blood pressure measurement in cats and the white coat effect. J Vet Intern Med 1999; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 14. Directive 86/609/EEC. Of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. [Google Scholar]
- 15. Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007; 17: 571–582. [DOI] [PubMed] [Google Scholar]
- 16. Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med 2007; 21: 402–409. [DOI] [PubMed] [Google Scholar]
- 17. Jepson R. Feline systemic hypertension classification and pathogenesis. J Feline Med Surg 2011; 13: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurtz TW, Griffin KA, Bidani AK, et al. Recommendations for blood pressure measurement in humans and experimental animals. Hypertension 2005; 45: 299–310. [DOI] [PubMed] [Google Scholar]
- 19. Brockway BP, Mills PA, Azar SH. A new method for continuous chronic measurement and recording of blood pressure, heart rate and activity in the rat via radio-telemetry. Clin Exp Hypertens A 1991; 13: 885–895. [DOI] [PubMed] [Google Scholar]
- 20. Truett AA, West DB. Validation of a radiotelemetry system for continuous blood pressure and heart rate monitoring in dogs. Lab Anim Sci 1995; 45: 299–302. [PubMed] [Google Scholar]
- 21. Schnell CR, Wood JM. Measurement of blood pressure and heart rate by telemetry in conscious, unrestrained marmosets. Am J Physiol 1993; 264: H1509–H1516. [DOI] [PubMed] [Google Scholar]
- 22. Sadoff DA, Fischel RJ, Carroll ME, et al. Chronic blood pressure radiotelemetry in rhesus macaque. Lab Anim Sci 1992; 42: 78–80. [PubMed] [Google Scholar]
- 23. Mitchell AZ, McMahon C, Beck TW, Sarazan RD. Sensitivity of two non-invasive blood pressure measurement techniques compared to telemetry in cynomolgus monkeys and beagle dogs. J Pharmacol Toxicol Methods 2010; 62:54–63. [DOI] [PubMed] [Google Scholar]
- 24. Brown S, Langford K, Tarver S. Effects of pharmacological agents on diurnal pattern of blood pressure, heart rate, and motor activity in cats. Am J Vet Res 1997; 58: 647–652. [PubMed] [Google Scholar]
- 25. Safar M.E, Laurent P. Pulse pressure and arterial stiffness in rats: comparison with humans. Am J Physiol Heart Circ Physiol 2003; 285: H1363–H1369. [DOI] [PubMed] [Google Scholar]
- 26. Safar M.E, Jankowski P. Central blood pressure and hypertension: role in cardiovascular risk assessment. Clin Sci 2009; 116: 273–282. [DOI] [PubMed] [Google Scholar]
- 27. Tsoucaris D, Benetos A, Legrand M, et al. Proximal and distal pulse pressure after acute antihypertensive vasodilating drugs in Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens 1995; 13: 243–249. [PubMed] [Google Scholar]
- 28. Godfraind T. Calcium antagonists and vasodilatation. Pharmacol Ther 1994; 64: 37–75. [DOI] [PubMed] [Google Scholar]
- 29. Xu B, Xiao-hong L, Lin G, et al. Amlodipine, but not verapamil or nifedipine, dilates rabbit femoral artery largely through a nitric oxide- and kinin-dependent mechanism. Br J Pharmacol 2002; 136: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brooks D, Horner RL, Kozar LF. Telemetry for long-term measurement of blood pressure. J Appl Physiol 1996; 81: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 31. Jepson RE, Hartley V, Mendl M, et al. A comparison of CAT Doppler and oscillometric Memoprint machines for non-invasive blood pressure measurement in conscious cats. J Feline Med Surg 2005; 7: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer O, Jenni R, Greiter-Wilke A, et al. Comparison of telemetry and high-definition oscillometry for blood pressure measurements in conscious dogs: effects of torcetrapib. JAALAS 2010; 49: 464–471. [PMC free article] [PubMed] [Google Scholar]
- 33. Bramlage CP, Schlumbohm C, Pryce CR, et al. Prenatal dexamethasone exposure does not alter blood pressure and nephron number in the young adult marmoset monkey. Hypertension 2009; 54: 1115–1122. [DOI] [PubMed] [Google Scholar]