Abstract
In several settings, the COVID-19 pandemic determined a negative impact on the occurrence of healthcare-associated infection, particularly for on central lines associated bloodstream infections (CLABSI). In our setting, we observed a significant increase in CLABSI in our intensive care unit (ICU) during 2020 and 2021 vs. 2018 to 2019. A refresher training activity on central venous catheter (CVC) management bundles was carried out in September–October 2021 for the ICU health staff. We assessed the impact of bundle implementation by means of standardized indicators, such as the Device Utilization Ratio (DUR), in this case, the Central Line Utilization Ratio, the Standardized Utilization Ratio (SUR), and the device Standardized Infection Ratio (dSIR). Standardized ratios for device use and infection ratio were computed using data from 2018 and 2019 as expectation data. After bundle implementation, we observed a significant reduction of dSIR (p < 0.001), which dropped from 3.23 and 2.99 in the 2020–2021 biennium to 1.11 in 2022 (CLABSI in the first quarter only); no more CLABSI were observed afterwards. Standardized ratios proved helpful in identify increasing trends of CLABSI in the ICU and monitoring the impact of a simple effective tool, i.e., training on and implementation of a bundle for CVC management.
Keywords: device Standardized Infection Ratio (dSIR), central lines associated bloodstream infections (CLABSI), intensive care units, Standardized Utilization Rate (SUR)
1. Introduction
Healthcare-associated infections (HAIs) pose a significant challenge in modern healthcare, contributing to increased morbidity, mortality, and healthcare costs [1,2]. These infections have a profound impact on intensive care units (ICUs), where patients are often critically ill and more susceptible to infections [3].
Antimicrobial stewardship programs and surveillance activities have emerged as key strategies in managing HAIs and improving patients’ outcomes by optimizing antimicrobial use, reducing microbial resistance, and decreasing the spread of infections caused by multidrug-resistant organisms (MDROs) [4]. Previous research has demonstrated both positive and neutral impacts of antimicrobial stewardship on HAIs incidence and prevalence [5].
Surveillance programs for HAIs are an essential component in monitoring HAIs incidence. Furthermore, by promptly identifying the extent and characteristics of an outbreak, such programs may reduce the subsequent incidence of HAIs [6]. The impact of outbreaks and ongoing diffusion of HAIs could be efficiently contrasted by implementing specific bundles (i.e., structured evidence-based procedures typically comprising three to five components that, when executed collectively and consistently, have been demonstrated to enhance patient outcomes) [7].
Bundles have proven to be effective in clinical practice for the purpose of preventing central lines associated bloodstream infections (CLABSIs). In 2006, Provonost et al. published an evidence-based intervention that resulted in a large and sustained reduction in CLABSIs in ICU [8]. During recent years, several authors from different countries reported similar results, thus confirming the role of such bundles [7,9,10,11]. However, adherence to bundles in antimicrobial stewardship is challenging to measure [12,13]. Despite these difficulties, several studies have shown adherence to these bundles could significantly improve patient outcomes [7,9,11,14,15,16].
In several settings, the COVID-19 pandemic had a negative impact on the adherence of healthcare staff to infection control measures and on circulation of MDROs in the same settings. This is evident from several reports from a large number of countries [17,18,19,20,21,22,23,24]. As known, the strategies to prevent CLABSI are linked to correct insertion and maintenance of the device [25]. Actually, as previously mentioned, several infection control strategies were (partially or largely) neglected during the pandemic, in view of the high number of patients admitted in serious conditions, within a short time frame and during phases of experienced staff shortage and increased turnover of both patients and staff [24]. It should also be pointed out Italy was, chronologically, the second nation to undergo the early impact of the COVID-19 pandemic. During the early stages of the pandemic, the effectiveness of treatments lacked high-level scientific evidence, resulting in frequent negative outcomes for many patients [26]. In this critical situation, the adequate management techniques for catheters, such as the maintenance of line dressing integrity and the hub scrub with chlorhexidine, received less attention due to the increased workload associated with critically ill COVID-19 patients.
In our setting, we observed an apparent increase in the incidence of CLABSI in ICU where a large proportion of patients were affected by COVID-19, thus it was decided to study the phenomenon and make specific interventions targeting the correct implementation of bundles for central vein catheters management.
Thus, the objective of our study is to assess, through the Standardized Infection Rate (SIR) and Standardized Utilization Ratio (SUR), the effectiveness of the bundles in decreasing the escalated occurrence of CLABSI events during the COVID-19 pandemic period in a single Italian ICU [27].
2. Results
We observed a total of 1679 admissions to ICU over a 5-year period (from 1 January 2018 to 31 December 2022). The number of admissions per year was not stable as it declined from the first, pre-COVID-19, period (2018–2019) to the second, pandemic, period (2020–2021). This reduction was the result of a significant decrease in surgical procedures and the necessity to admit patients affected by severe COVID-19 pneumonia to the ICU. The overall duration of patients’ stays in the ICU increased from the pre-COVID-19 to pandemic period (Table 1). Actually, the different type of patients admitted to the ICU determined an increase in the average length of stay of patients from the first (6.09 and 6.03 days) to the second period (7.84 and 8.45 days).
Table 1.
Number of patients, total intensive care unit (ICU) days, and total central line days by year of study surveillance.
| Year | Number of Patients | Patients Stay in ICU (Total Days) | Central Line Days |
|---|---|---|---|
| 2018 | 375 | 2284 | 1716 |
| 2019 | 371 | 2238 | 1867 |
| 2020 | 337 | 2643 | 2221 |
| 2021 | 309 | 2613 | 1915 |
| 2022 | 287 | 2252 | 1932 |
Moreover, following the onset of the COVID-19 pandemic, we observed an increase in the use of central lines (Table 1). Although incidence of SARS-CoV-2 infections significantly decreased in 2022, the use of central lines remained high, while the number of patients treated in 2022, as well as total stay of patients in ICU, were similar to the 2020–2021 biennium.
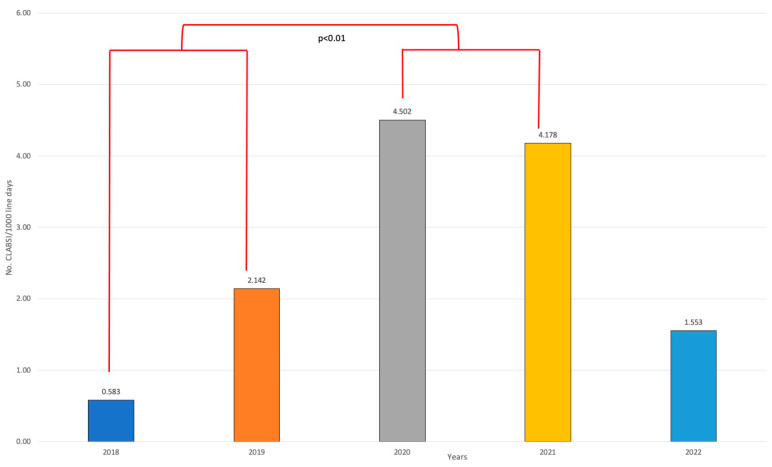
CLABSI incidence increased from the first period to the second one; this was followed by a drastic reduction in 2022 (63% less than 2021) (Figure 1). CLABSI incidence significantly increased from the pre-pandemic years 2018–2019 to the intra-pandemic years 2020–2021 (p < 0.01 chi-square test).
Figure 1.
Central lines associated bloodstream infections (CLABSI) incidence per year during study period.
The average time between central venous catheter (CVC) insertion and the onset of CLABSI was 12 days. This timing did not change over the study period, with the exception of two cases of CLABSI, which occurred shortly after CVC implantation.
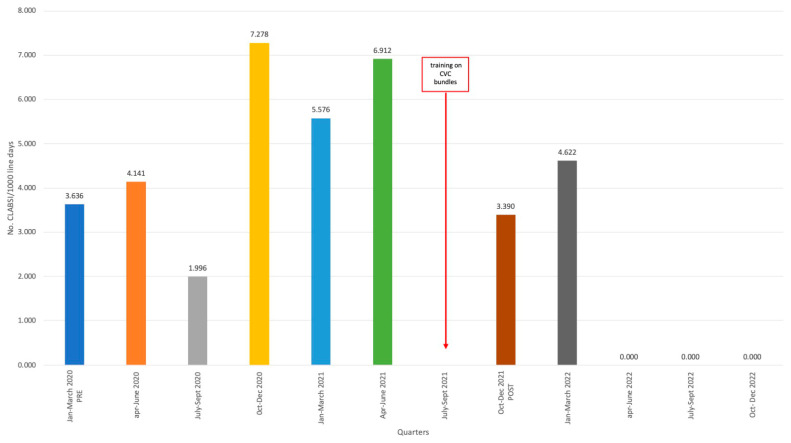
In light of the persistence of a relatively elevated incidence of CLABSIs during 2022, a quarter-by-quarter assessment was carried out starting from the first quarter of 2020. The July–September 2021 quarter was not included because all training activities were carried out during it, and there could have been a bias in performance. This analysis revealed this persistently increased incidence was due to several events occurring in the first quarter of 2022. Subsequently, the incidence reached zero and remained stable during the following three quarters (Figure 2).
Figure 2.
Incidence of Central lines associated bloodstream infections (CLABSI) broken down by quarters from 2020 to 2022.
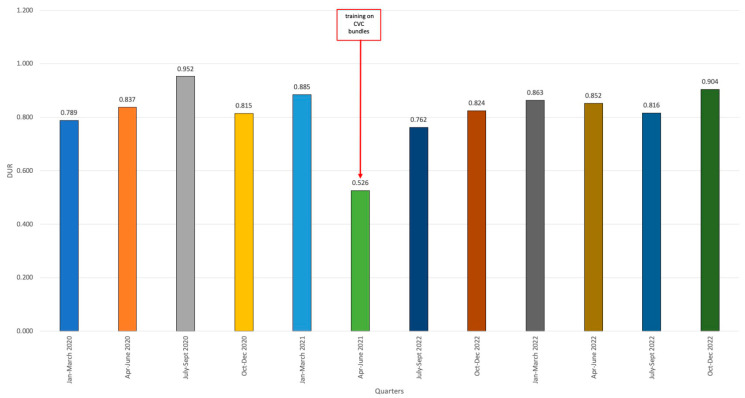
Regarding CVC utilization, a variable rate of intravascular device utilization was observed. When compared to the pre-COVID period (2018–2019), DUR was lower, and remained stable over the pandemic years. This could lead to the assumption that the device use was lower during the pandemic. This reduction was especially pronounced in the second quarter of 2021, reaching 0.526 (Figure 3).
Figure 3.
Device Utilization Ratio (DUR) values by quarters between the last quarter of 2020 and the last quarter of 2022.
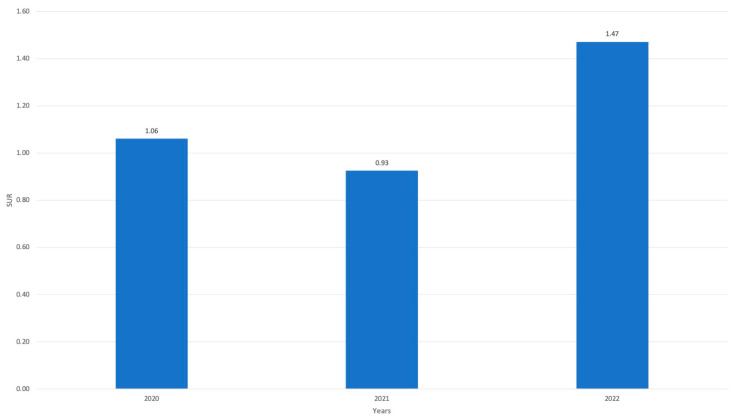
However, when adjusted for the aforementioned factors, and therefore calculating the correspondent standardized value (SUR), we observed the use of CVC remained stable during the pandemic period when compared to the pre-COVID-19 period, with values just slightly different from 1.0. Following the end of the pandemic period, during 2022, and despite the reduction of patients hospitalized in the ICU, the SUR figures rose to 1.47, demonstrating a clear increase in CVC utilization when adjusted for the correct variables (Figure 4).
Figure 4.
Standardized Utilization Ratio (SUR) values during 2020, 2021, and 2022 in comparison with 2018 and 2019.
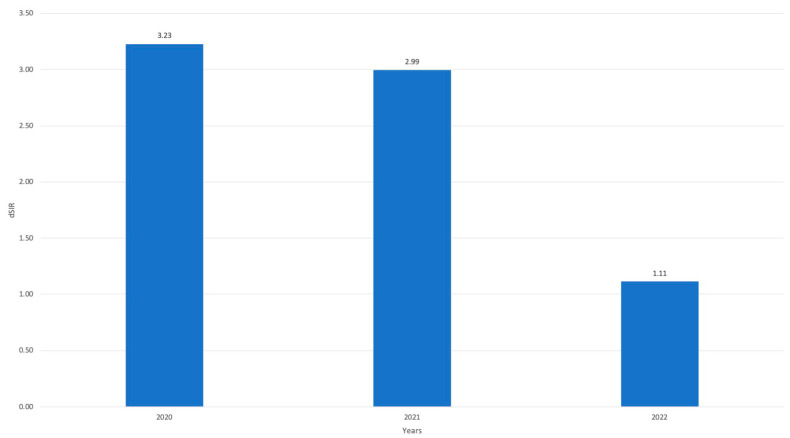
After acquiring the standardized measurement of CVC utilization, we undertook the computation of the trend in the occurrence of CLABSI. This analysis was adjusted for the utilization of the device, thereby enabling us to derive the dSIR value. The dSIR values, not surprisingly, exceeded the projected value, reaching 3.23 in 2020 and 2.99 in 2021. Nevertheless, the decline detected through incidence analysis in 2022 was inadequate to attain a value below 1.0. Even though it stood at 1.11, marginally surpassing the anticipated pre-pandemic values. Nevertheless, the difference between the pandemic period and 2022 reached a statistically significant variation (p < 0.001). (Figure 5).
Figure 5.
Standardized Infection Rate (SIR) adjusted for the utilization of CVC (dSIR) calculated over the three-year period 2020–2022, based on the expected figures estimated from 2018–2019.
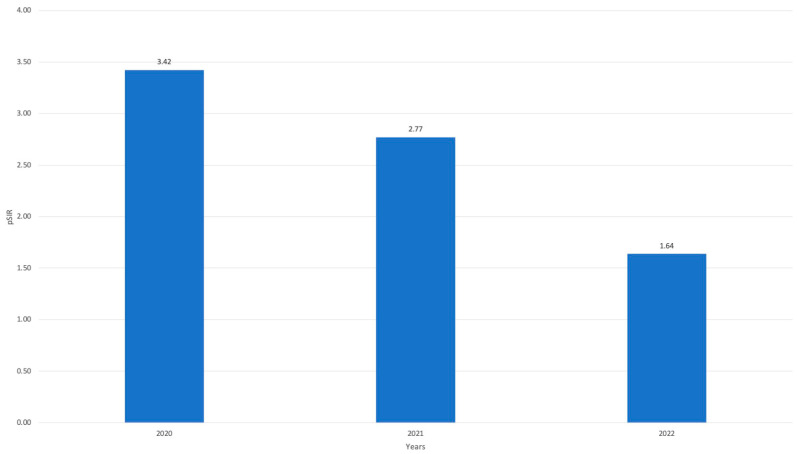
The population SIR (pSIR) was subsequently calculated using the dSIR to assess the CLABSI trend through a population analysis. Even in this particular case, the pSIR values (which were determined based on both the dSIR and SUR) were higher throughout the duration of the pandemic (3.42 in 2020, 2.77 in 2021), with a decrease observed in 2022, albeit to a lesser extent than that calculated using the dSIR (1.64) (Figure 6). This may be due to the nature of our intervention, as we designed it to ameliorate the management of the CVC, not to reduce its use.
Figure 6.
Population SIR (pSIR) by year from 2020 to 2022.
Intervention
We directly observed device insertions twice with 100% adherence to the insert device bundle. We decided to observe a limited number of insertions because our retrospective evaluation of CLABSI identified a really low number of early ones. Differently, since the median time to CLABSI onset was of 12 days, we assumed the major difficulties were in CVC management rather than insertion. Thus, we observed more frequently CVC management. Observations of CVC management occurred six times, with registration of observation or lack of observation of the different bundle items. In particular, the items that were most adhered to were “the intact dressing and replacing every 7 days” (100%), while those that were least adhered to were “hub scrub with 2% chlorhexidine in 70% isopropyl alcohol” (33.33%). The observations evaluated the adherence to each single bundle point; 1 point was given for each bundle that was respected, 0 point if the bundle was not respected. The overall bundle adherence rate during observation visits was 78.12%.
3. Discussion
COVID-19 has caused an increase in the complexity of clinical management of patients in ICU, thus determining both an increase in resources used and in patient stay [28,29,30]. The surveillance of the incidence of CLABSI revealed a negative impact of SARS-CoV-2 pandemic also in our setting, resulting in a statistically significant increase in CLABSI incidence in our ICU.
A lot of studies identified several potential factors that could have affected the observed increased circulation of MDROs and increased incidence of CLABSI and other infections in the ICU during COVID-19 pandemic. Some factors, although not all unanimously confirmed, may have also contributed to the observed increase in patient complexity and length of stay in ICU. They include, for example, overcrowding of intensive care units, prolonged and repeated patient pronation, improper/inadequate use of protective devices, alteration in infection control procedures, treatment of immunomodulating agents (e.g., tocilizumab, sarilumab, anakinra, etc), longer ICU stays, recruitment of untrained personnel, and even in the incidence of infections caused by MDROs [24,27,31,32,33,34,35,36,37,38,39,40]. In particular, the activity of proning patients to improve their respiratory function by improving lung recruitment and better lung ventilation/perfusion matching has proven to be an important determinant, significantly associated with increased colonization by Carbapenem-Resistant Enterobacteriaceae (CRE), device loss or traction, and bacteremia [27,31,35,41,42,43,44]. The increased rate of intravascular device use reported with the pandemic onset could lead to the conclusion that the increased incidence of CLABSI may be due not only to the increased complexity of patients admitted to the ICU during the COVID-19 pandemic, but also to the lack of adherence to infection control measures. Actually, the observed overcrowding in the ICU, together with trained staff shortages, may have caused a reduction in the frequency of contacts with patients and in CVC maintenance (e.g., chlorhexidine bathing, scrubbing the hub, site examinations) as well as disruptions in processes of care (e.g., risking disrupting catheter dressings when placing patients in a prone position), thus contributing to an increase in CLABSI incidence in this setting [27,34,37,45]. Another significant aspect was the changed feature of patients hospitalized in the ICU. During the COVID-19 pandemic, at least initially, admission policies in ICUs changed due to limitations in major surgery activities, thus privileging COVID-19 patients with consequent limited access to less severe cases. Thus, the increase in CLABSI rates could be due to the decrease in the denominator, which was primarily composed of patients with lower CLABSI risk [34,37].
In our experience, amidst the COVID-19 pandemic, we came across multiple potentially significant events in the ICU. In particular, we observed a decline in the number of hospitalized patients, an increase in the average duration of patients’ stays, and a surge in the utilization of CVCs. In our experience, average patients’ ICU stays exhibited a decline in 2022, following the reduction of COVID-19 pneumonia cases, while CVC use remained elevated. Concerning CLABSI incidence, we observed a significant rise in incidence during the pandemic period. This situation, in September 2021, led to the training interventions designed to improve the CVC management. Subsequently, a ‘refreshed’ implementation of CVC bundles was carried out. Following the intervention, the occurrence of CLABSIs remained stable for two quarters, and then receded after the second quarter of 2022.
To standardize the data and mitigate confounding biases associated with different patient types and therapeutic strategies related to the insertion of the CVC across distinct time periods (pandemic and post-pandemic), we have conducted an analysis utilizing unstandardized (DUR) and standardized ratios (SUR, dSIR, and pSIR). The DUR, which represented the device utilization ratio, revealed a consistent or potentially decreased utilization of CVC in comparison to the reference period of 2018–2019 (serving as a benchmark for our prediction).
The SUR, nonetheless, ascertained, upon standardization, the data revealed considerable stability in CVC use, for which we observed an increase in 2022 as a result of the deflating of the pandemic. The dSIR and the pSIR identified a noteworthy decrease in the occurrence of CLABSI, even after standardization for the device’s utilization and the category of hospitalized patient, thereby demonstrating CLABSI reduction was not associated with different categories of patients or with reduced device use.
We can conclude our study showed the potential for rapid favourable outcomes associated with the utilization of bundles in ICU that target CVC management. Our study confirms existing data in scientific literature concerning bundles’ efficacy in reducing CLABSI incidence [16,46,47]. Nonetheless, our study has additionally provided valuable insights derived from standardized data, including the incidence of CLABSI, the components encompassed within the bundle, and the level of compliance exhibited by healthcare personnel towards adhering to these components. Our application of standardized ratios for the analysis of incidence has additionally facilitated the alleviation of selection bias effects, allowing for a more precise analysis compared to a relatively raw incidence analysis.
3.1. Limitations
The main limitations of this study is that the investigation was carried out in a single ICU, only. Other limitations include the observational and retrospective nature of the study, including data collection and evaluation of the impact of the training activity. Such limitations may have had a limited impact since the main epidemiological findings were coherent with current literature. In addition, the impact of training activity and of the subsequent implementation of CVC bundles is in accordance with what is reported in the literature in the pre-COVID-19 era.
3.2. Future Implications
The exercise of collecting local epidemiological data is relatively easy, but the information from raw data can be misleading. The use of standardized ratios helped us to properly compare data from quarter to quarter, from year to year, independently from the changing epidemiological landscape and patient types in the ICU. The use of standardized ratios may help in comparing epidemiological findings with other regional, national, or international data/studies. Their diffusion and their sharing in scientific publications could be really helpful in comparing our findings with data from other settings.
Other messages for the future are to keep looking at the basics of infection control and to remember that key simple interventions such as training, bundles implementations, and monitoring can be extremely cost-effective.
4. Materials and Methods
The study is a retrospective evaluation of CLABSI incidence in a northern Italian nationally renowned and highly specialized hospital organized by treatment intensity. The aforementioned hospital consists of three buildings and accommodates 458 beds, primarily in 3- and 4-bed rooms, with over 15,000 routine admissions annually, along with more than 8600 medical procedures conducted in outpatient and day surgery settings. The ICU within the hospital is designed as an open space, incorporating two isolation rooms, collectively offering 8 beds. Furthermore, the ICU was equipped with the capability to expand its capacity to accommodate up to 12 beds, as it frequently happened during COVID-19 epidemic peaks.
4.1. Population
We included all hospitalized patients in the ICU who had an episode of CLABSI from 1 January 2018 to 31 December 2022 (5 years of observation).
The CLABSI events were identified according to CDC definition, and the data were collected, including device, patients’ days and microorganisms’ infection-related data [48]. Only CLABSI attributable to the ICU, in accordance with CDC criteria, were analyzed.
We calculated the incidence of CLABSI (CLABSI number/line days) by dividing it into both quarters and years. Analyses were performed for both cases. To give a better understanding of the intervention’s efficacy, the Device Utilization Ratio (DUR), in this case, the Central Line Utilization Ratio (DUR), the Standardized Utilization Ratio (SUR), and the device Standardized Infection Ratio (dSIR) were used as tools for analyzing trends of HAIs [49,50]. These metrics provide valuable insights into the incidence and prevalence of HAIs, aiding in the evaluation and improvement of infection control measures.
As the DUR defines but does not standardize the degree of device utilization, SUR is theoretically more informative, as it standardizes the measure obtained with DUR by adjusting it for various facility and/or location-level factors that contribute to device use [50]. On the other hand, dSIR is a standardized measure used to track HAIs at a national, state, or local level over time and can be used to measure progress from a single point in time [49]. Values obtained are normally compared with benchmarks (in our case, are based on the expectations from the previous data of the ICU during the 2018–2019 biennium). A result greater than 1.0 indicates the events were superior to what is predicted; conversely, a value inferior to 1.0 indicates events were fewer than predicted.
A key component of device risk reduction is decreasing exposure to the device, either by preventing its insertion or reducing its duration of use. By examining both dSIR (reflecting rate of infection and device use) and SUR (reflecting device use), the impact of interventions can be measured. It should be noted changes in dSIR may also occur if the interventions result in a significant difference in catheter use and frequently underestimate improvement in infection rates, mostly because they fail to account for reduced device utilization associated with infection prevention intervention. To overcome this problem, Fakih MG et al. in their 2019 paper proposed the use of the population SIR (pSIR), which combined the device SIR (dSIR) and the standardized device use ratio (SUR) [51]. Thus, the pSIR refers to the entire population, adjusted for expected device use. The value of the pSIR is calculated as dSIR*SUR.
4.2. Description of Training on CVC Bundles
All ICU staff were trained with a specific course on HAIs, including formal lectures on principles of HAIs and specific bundles, and hands-on practice on a training dummy.
Specific training activities targeting CVCs in our program were based on the introduction and application in clinical practice of bundles regarding actions ranging from the insertion to the management of CVCs. A newly developed manual for bundles at our institution (Available at: https://www.galliera.it/20/58/strutture-e-servizi-in-staff-alla-direzione-sanitaria/858/io-e-manuali-cio/manuale-operativo-mo/bundle-manuale-per-la-prevenzione-delle-ica/view, (accessed on 19 December 2023)) was used for such training activities. The key issues included in the manual and discussed during the training were:
device insertion,
guided ultrasound procedure,
surgical hand washing aseptic technique,
skin antisepsis with 2% chlorhexidine in 70% isopropyl alcohol,
use of sutureless fixation device,
management,
hand washing with alcohol solution before and after using the catheter,
hub scrub with chlorhexidine,
keep the dressing intact and replace it every 7 days,
remove as soon as possible.
In addition, washable keepsake posters based on the new bundles’ manual were developed and affixed throughout the hospital, including the ICU.
Based on CLABSI surveillance data from 2020 and first quarters of 2021, improvement actions were planned and implemented from September 2021 to October 2021. The training activities involved 44 nurses over one month.
Ten safety walks were carried out in the ICU to promote culture safety and raise awareness among operators regarding the rise in CLABSI incidence.
Starting from January 2022, six direct observations were carried out to evaluate bundles’ adherence.
4.3. Statistical Analysis
All patient characteristics are presented as mean with standard deviation, median, and range for continuous variables, and expressed as absolute values along with percentages for categorical variables. The Chi-squared test was used to assess independence between variables. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Stata/SE 18 software (StataCorp LP, College Station, TX, USA).
5. Conclusions
When faced with the COVID-19 pandemic and the increase in difficulties associated with patient management, coupled with the subsequent surge in CLABSI incidence in the ICU, conducting an investigation based on standardized information and the implementation of bundled interventions held the capacity to detect a problem and elicit a relatively swift response aimed at curtailing the incidence of CLABSI.
Most of our findings were in line with the international literature. However, the key aspect of our study was that we always needed to go beyond our, even scientifically sound, observations. We need to find solutions to the detected problems, and we proved that sometimes relatively easy solutions existed to overcome worrisome situations. In addition, we proved, by means of standardized indicators, the effectiveness of the introduced intervention. Investing in people (training on bundles) showed to be extremely effective in reducing life-threatening infections (CLABSIs in ICU).
Abbreviations
| HAIs | Healthcare-associated infections |
| ICUs | intensive care units |
| MDROs | multidrug-resistant organisms |
| CLABSIs | central lines associated bloodstream infections |
| COVID-19 | coronavirus disease |
| SIR | Standardized Infection Rate |
| SUR | Standardized Utilization Rate |
| dSIR | Device Standardized Infection Rate |
| CVCs | central venous catheters |
| DUR | Device Utilization Rate |
| pSIR | population Standardized Infection Rate. |
Author Contributions
Conceptualization, S.B.; methodology, M.S.; validation, M.L.C. and E.P.; formal analysis, M.S.; investigation, M.S. and F.D.P.; resources, G.A., E.B.V., N.B., M.F., V.G., M.L., M.N., A.P., R.P., M.C.S. and S.T.; data curation, M.S., A.C. and M.O.; writing—original draft preparation, S.B., M.S. and F.D.P.; writing—review and editing, S.B., M.S., F.D.P., G.A., E.B.V., N.B., A.C., M.F., V.G., M.L., M.N., M.O., A.P., R.P., M.C.S., S.T., M.L.C. and E.P.; visualization, F.D.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study because all analyzed data were extracted anonymously by the laboratory and the study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to internal regulations.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Burchardi H., Schneider H. Economic aspects of severe sepsis: A review of intensive care unit costs, cost of illness and cost effectiveness of therapy. PharmacoEconomics. 2004;22:793–813. doi: 10.2165/00019053-200422120-00003. [DOI] [PubMed] [Google Scholar]
- 2.Tiru B., DiNino E.K., Orenstein A., Mailloux P.T., Pesaturo A., Gupta A., McGee W.T. The Economic and Humanistic Burden of Severe Sepsis. PharmacoEconomics. 2015;33:925–937. doi: 10.1007/s40273-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 3.Mathur P., Malpiedi P., Walia K., Srikantiah P., Gupta S., Lohiya A., Chakrabarti A., Ray P., Biswal M., Taneja N., et al. EIndian Healthcare Associated Infection Surveillance Network collaborators. Health-care-associated bloodstream and urinary tract infections in a network of hospitals in India: A multicentre, hospital-based, prospective surveillance study. Lancet Glob. Health. 2022;10:e1317–e1325. doi: 10.1016/S2214-109X(22)00274-1. [DOI] [PubMed] [Google Scholar]
- 4.MacDougall C., Polk R.E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005;18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertollo L.G., Lutkemeyer D.S., Levin A.S. Are antimicrobial stewardship programs effective strategies for preventing antibiotic resistance? A systematic review. Am. J. Infect. Control. 2018;46:824–836. doi: 10.1016/j.ajic.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Sticchi C., Alberti M., Artioli S., Assensi M., Baldelli I., Battistini A., Boni S., Cassola G., Castagnola E., Cattaneo M., et al. Collaborative Group for the Point Prevalence Survey of healthcare-associated infections in Liguria. Regional point prevalence study of healthcare-associated infections and antimicrobial use in acute care hospitals in Liguria, Italy. J. Hosp. Infect. 2018;99:8–16. doi: 10.1016/j.jhin.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Gupta P., Thomas M., Patel A., George R., Mathews L., Alex S., John S., Simbulan C., Garcia M.L., Al-Balushi S., et al. Bundle approach used to achieve zero central line-associated bloodstream infections in an adult coronary intensive care unit. BMJ Open Qual. 2021;10:e001200. doi: 10.1136/bmjoq-2020-001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pronovost P., Needham D., Berenholtz S., Sinopoli D., Chu H., Cosgrove S., Sexton B., Hyzy R., Welsh R., Roth G., et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N. Engl. J. Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Aceituno A., Vega-Costa V., Ruiz-Álvarez M., Figuerola-Tejerina A., Méndez-Hernández R., Ramasco-Rueda F. Effectiveness of a bundle of measures for reducing central line-associated bloodstream infections. Rev. Esp. Anestesiol. Reanim. 2020;67:227–236. doi: 10.1016/j.redar.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Goldman J., Rotteau L., Shojania K.G., Baker G.R., Rowland P., Christianson M.K., Vogus T.J., Cameron C., Coffey M. Implementation of a central-line bundle: A qualitative study of three clinical units. Implement. Sci. Commun. 2021;2:105. doi: 10.1186/s43058-021-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama M.F., Jamal W., Al Mousa H., Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J. Infect. Public Health. 2016;9:34–41. doi: 10.1016/j.jiph.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Ray-Barruel G., Xu H., Marsh N., Cooke M., Rickard C.M. Effectiveness of insertion and maintenance bundles in preventing peripheral intravenous catheter-related complications and bloodstream infection in hospital patients: A systematic review. Infect. Dis. Health. 2019;24:152–168. doi: 10.1016/j.idh.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Barker A.K., Ngam C., Musuuza J.S., Vaughn V.M., Safdar N. Reducing Clostridium difficile in the Inpatient Setting: A Systematic Review of the Adherence to and Effectiveness of C. difficile Prevention Bundles. Infect. Control. Hosp. Epidemiol. 2017;38:639–650. doi: 10.1017/ice.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettit N.N., Han Z., Nguyen C.T., Choksi A., Charnot-Katsikas A., Beavis K.G., Tesic V., Pisano J. Antimicrobial Stewardship Review of Automated Candidemia Alerts Using the Epic Stewardship Module Improves Bundle-of-Care Adherence. Open Forum Infect. Dis. 2019;6:ofz412. doi: 10.1093/ofid/ofz412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathi S., Pallotto E., Hord J., Staubach K., Sisso P., Mack E., Coffey M. 1048: Association of bundle compliance with CLABSI rates: A solutions for patient safety (sps) report. Crit. Care Med. 2022;51:518. doi: 10.1097/01.ccm.0000909920.98948.16. [DOI] [Google Scholar]
- 16.Simoneaux C., Guerra P. Implementation of Evidence-based Maintenance Bundles to Reduce Central Line-Associated Bloodstream Infection (CLABSI) and Catheter-Associated Urinary Tract Infection (CAUTI) Rates. Am. J. Infect. Control. 2022;50:S34. doi: 10.1016/j.ajic.2022.03.057. [DOI] [Google Scholar]
- 17.Kampmeier S., Tönnies H., Correa-Martinez C.L., Mellmann A., Schwierzeck V. A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob. Resist. Infect. Control. 2020;9:154. doi: 10.1186/s13756-020-00820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks S.K., Greenberg N., Wessely S., Rubin G.J. Factors affecting healthcare workers’ compliance with social and behavioural infection control measures during emerging infectious disease outbreaks: Rapid evidence review. BMJ Open. 2021;11:e049857. doi: 10.1136/bmjopen-2021-049857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abed Alah M., Abdeen S., Selim N., Hamdani D., Radwan E., Sharaf N., Al-Katheeri H., Bougmiza I. Compliance and barriers to the use of infection prevention and control measures among health care workers during COVID-19 pandemic in Qatar: A national survey. J. Nurs. Manag. 2021;29:2401–2411. doi: 10.1111/jonm.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirabayashi A., Kajihara T., Yahara K., Shibayama K., Sugai M. Impact of the COVID-19 pandemic on the surveillance of antimicrobial resistance. J. Hosp. Infect. 2021;117:147–156. doi: 10.1016/j.jhin.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Carvalho Hessel Dias V.M., Tuon F., de Jesus Capelo P., Telles J.P., Fortaleza C.M.C.B., Pellegrino Baena C. Trend analysis of carbapenem-resistant Gram-negative bacteria and antimicrobial consumption in the post-COVID-19 era: An extra challenge for healthcare institutions. J. Hosp. Infect. 2022;120:43–47. doi: 10.1016/j.jhin.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weldetinsae A., Alemu Z.A., Tefaye K., Gizaw M., Alemahyehu E., Tayachew A., Derso S., Abate M., Getachew M., Abera D., et al. Adherence to infection prevention and control measures and risk of exposure among health-care workers: A cross-sectional study from the early period of COVID-19 pandemic in Addis Ababa, Ethiopia. Health Sci. Rep. 2023;6:e1365. doi: 10.1002/hsr2.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oweidat K.A., Toubasi A.A., Khraisat F.A., Aldahabi M.N., Alghrabli A., Khater Y., Saleh N., Al-Sayegh T.N., Albtoosh A.S. The Impact of COVID-19 on Antibiotic Resistance and Clinical Outcomes among Critically Ill Patients. Am. J. Infect. Control. 2023 doi: 10.1016/j.ajic.2023.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Parisini A., Boni S., Vacca E.B., Bobbio N., Puente F.D., Feasi M., Prinapori R., Lattuada M., Sartini M., Cristina M.L., et al. Impact of the COVID-19 Pandemic on Epidemiology of Antibiotic Resistance in an Intensive Care Unit (ICU): The Experience of a North-West Italian Center. Antibiotics. 2023;12:1278. doi: 10.3390/antibiotics12081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haque M., McKimm J., Sartelli M., Dhingra S., Labricciosa F.M., Islam S., Jahan D., Nusrat T., Chowdhury T.S., Coccolini F., et al. Strategies to Prevent Healthcare-Associated Infections: A Narrative Overview. Risk Manag. Healthc. Policy. 2020;13:1765–1780. doi: 10.2147/RMHP.S269315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sartini M., Del Puente F., Oliva M., Carbone A., Blasi Vacca E., Parisini A., Boni S., Bobbio N., Feasi M., Battistella A., et al. Riding the COVID Waves: Clinical Trends, Outcomes, and Remaining Pitfalls of the SARS-CoV-2 Pandemic: An Analysis of Two High-Incidence Periods at a Hospital in Northern Italy. J. Clin. Med. 2021;10:5239. doi: 10.3390/jcm10225239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakih M.G., Bufalino A., Sturm L., Huang R.H., Ottenbacher A., Saake K., Winegar A., Fogel R., Cacchione J. Coronavirus disease 2019 (COVID-19) pandemic, central-line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts. Infect. Control. Hosp. Epidemiol. 2022;43:26–31. doi: 10.1017/ice.2021.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruyneel A., Gallani M.C., Tack J., d’Hondt A., Canipel S., Franck S., Reper P., Pirson M. Impact of COVID-19 on nursing time in intensive care units in Belgium. Intensive Crit. Care Nurs. 2021;62:102967. doi: 10.1016/j.iccn.2020.102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L., Xu J., Wu Y., Huang C., Ouyang Y., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit. Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiner-Lastinger L.M., Pattabiraman V., Konnor R.Y., Patel P.R., Wong E., Xu S.Y., Smith B., Edwards J.R., Dudeck M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control. Hosp. Epidemiol. 2022;43:12–25. doi: 10.1017/ice.2021.362. [DOI] [PubMed] [Google Scholar]
- 31.Tiri B., Sensi E., Marsiliani V., Cantarini M., Priante G., Vernelli C., Martella L.A., Costantini M., Mariottini A., Andreani P., et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020;9:2744. doi: 10.3390/jcm9092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pontali E., Volpi S., Signori A., Antonucci G., Castellaneta M., Buzzi D., Montale A., Bustaffa M., Angelelli A., Caorsi R., et al. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J. Allergy Clin. Immunol. 2021;147:1217–1225. doi: 10.1016/j.jaci.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyriazopoulou E., Huet T., Cavalli G., Gori A., Kyprianou M., Pickkers P., Eugen-Olsen J., Clerici M., Veas F., Chatellier G., et al. International Collaborative Group for Anakinra in COVID-19. Effect of anakinra on mortality in patients with COVID-19: A systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021;3:e690–e697. doi: 10.1016/S2665-9913(21)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMullen K.M., Smith B.A., Rebmann T. Impact of SARS-CoV-2 on hospital acquired infection rates in the United States: Predictions and early results. Am. J. Infect. Control. 2020;48:1409–1411. doi: 10.1016/j.ajic.2020.06.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tetaj N., Capone A., Stazi G.V., Marini M.C., Garotto G., Busso D., Scarcia S., Caravella I., Macchione M., De Angelis G., et al. ICU COVID-19 Study Group. Epidemiology of ventilator-associated pneumonia in ICU COVID-19 patients: An alarming high rate of multidrug-resistant bacteria. J. Anesth. Analg. Crit. Care. 2022;2:36. doi: 10.1186/s44158-022-00065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lastinger L.M., Alvarez C.R., Kofman A., Konnor R.Y., Kuhar D.T., Nkwata A., Patel P.R., Pattabiraman V., Xu S.Y., Dudeck M.A. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control. Hosp. Epidemiol. 2023;44:997–1001. doi: 10.1017/ice.2022.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alsaffar M.J., Alsheddi F.M., Humayun T., Aldalbehi F.Z., Alshammari W.H.S., Aldecoa Y.S., Burhan N.M., El-Saed A., Tawfeeq S., Alanazi K.H. Impact of COVID-19 pandemic on the rates of central line-associated bloodstream infection and catheter-associated urinary tract infection in an intensive care setting. A National experience. Am. J. Infect. Control. 2023;51:1108–1113. doi: 10.1016/j.ajic.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahrmann J.M., Nickel K.B., Stwalley D., Dubberke E.R., Lyons P.G., Michelson A.P., McMullen K.M., Gandra S., Olsen M.A., Kwon J.H., et al. Healthcare-associated infections (HAIs) during the coronavirus disease 2019 (COVID-19) pandemic: A time-series analysis. Antimicrob. Steward. Healthc. Epidemiol. 2023;3:e14. doi: 10.1017/ash.2022.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder G.M., Wagester S., Harris P.L., Valek A.L., Hodges J.C., Bilderback A.L., Kader F., Tanner C.A., Metzger A.P., DiNucci S.E., et al. Healthcare-associated infections during the coronavirus disease 2019 (COVID-19) pandemic and the modulating effect of centralized surveillance. Antimicrob. Steward. Healthc. Epidemiol. 2023;3:e72. doi: 10.1017/ash.2023.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sands K.E., Blanchard E.J., Fraker S., Korwek K., Cuffe M. Health Care-Associated Infections Among Hospitalized Patients With COVID-19, March 2020–March 2022. JAMA Netw. Open. 2023;6:e238059. doi: 10.1001/jamanetworkopen.2023.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scaramuzzo G., Gamberini L., Tonetti T., Zani G., Ottaviani I., Mazzoli C.A., Capozzi C., Giampalma E., Bacchi Reggiani M.L., Bertellini E., et al. ICU-RER COVID-19 Collaboration Sustained oxygenation improvement after first prone positioning is associated with liberation from mechanical ventilation and mortality in critically ill COVID-19 patients: A cohort study. Ann. Intensive Care. 2021;11:63. doi: 10.1186/s13613-021-00853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okin D., Huang C.Y., Alba G.A., Jesudasen S.J., Dandawate N.A., Gavralidis A., Chang L.L., Moin E.E., Ahmad I., Witkin A.S., et al. Prolonged Prone Position Ventilation Is Associated With Reduced Mortality in Intubated COVID-19 Patients. Chest. 2023;163:533–542. doi: 10.1016/j.chest.2022.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabiatti D., Vieira L.G., Margatho A.S., Dos Santos B.N., Clark A.M., Vasques C.I., Silveira R.C.C.P. Prevalence of adverse events in pronated intubated adult COVID-19 patients: A systematic review with meta-analysis. J. Clin. Nurs. 2023;33:58–75. doi: 10.1111/jocn.16741. [DOI] [PubMed] [Google Scholar]
- 44.Frattari A., Polilli E., Rapacchiale G., Coladonato S., Ianniruberto S., Mazzotta E., Patarchi A., Battilana M., Ciulli R., Moretta A., et al. Predictors of bacteremia and death, including immune status, in a large single-center cohort of unvaccinated ICU patients with COVID-19 pneumonia. Eur. J. Med. Res. 2023;28:219. doi: 10.1186/s40001-023-01166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel P.R., Weiner-Lastinger L.M., Dudeck M.A., Fike L.V., Kuhar D.T., Edwards J.R., Pollock D., Benin A. Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect. Control. Hosp. Epidemiol. 2022;43:790–793. doi: 10.1017/ice.2021.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menegueti M.G., Ardison K.M., Bellissimo-Rodrigues F., Gaspar G.G., Martins-Filho O.A., Puga M.L., Laus A.M., Basile-Filho A., Auxiliadora-Martins M. The Impact of Implementation of Bundle to Reduce Catheter-Related Bloodstream Infection Rates. J. Clin. Med. Res. 2015;7:857–861. doi: 10.14740/jocmr2314w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumyati G., Concannon C., van Wijngaarden E., Love T.M., Graman P., Pettis A.M., Greene L., El-Daher N., Farnsworth D., Quinlan G., et al. Sustained reduction of central line-associated bloodstream infections outside the intensive care unit with a multimodal intervention focusing on central line maintenance. Am. J. Infect. Control. 2014;42:723–730. doi: 10.1016/j.ajic.2014.03.353. [DOI] [PubMed] [Google Scholar]
- 48.CDC Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection) [(accessed on 16 August 2023)];2023 Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf.
- 49.CDC The NHSN Standardized Infection Ratio (SIR) A Guide to the SIR (Based on 2015 National Baseline) Updated April 2022. [(accessed on 16 August 2023)]; Available online: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sir-guide.pdf.
- 50.CDC THE NHSN Standardized Utilization Ratio (SUR) A Guide to the SUR (Based on 2015 National Baseline) Updated April 2022. [(accessed on 16 August 2023)]; Available online: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sur-guide-508.pdf.
- 51.Fakih M.G., Huang R.H., Bufalino A., Erlinger T., Sturm L., Hendrich A., Haydar Z. The case for a population standardized infection ratio (SIR): A metric that marries the device SIR to the standardized utilization ratio (SUR) Infect. Control. Hosp. Epidemiol. 2019;40:979–982. doi: 10.1017/ice.2019.175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to internal regulations.