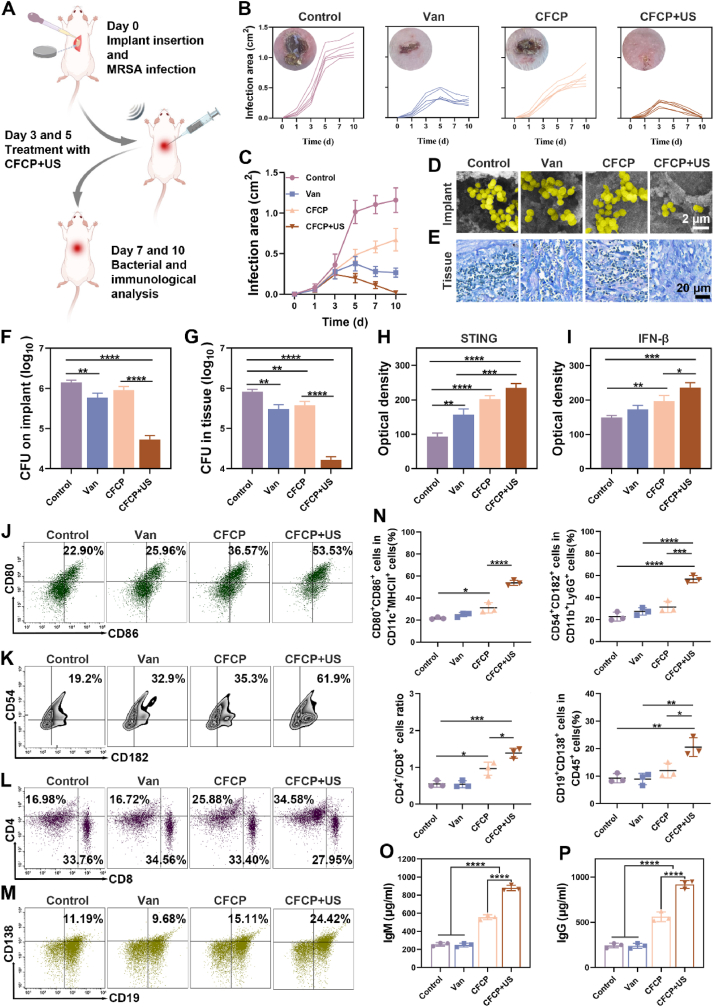
Fig. 6.
CFCP+US treatment activates systemic immune responses against primary IRIs in vivo. (A) Schematic diagram of the construction and intervention of the murine primary IRI model. (B) Curves of the individual infection areas at different time points for each treatment group and the representative photograph of wounds on day 10. (C) Curves of the average infection area in different treatment groups. (D) Representative SEM images of subcutaneous implants of mice with different treatments on day 10. Yellow for S. aureus. (E) Giemsa staining results of peri-implant tissues on day 10 from different treatment groups. (F and G) Bacterial counts of implants and peri-implant tissues on day 10 as determined by SPM. (H and I) Quantification of STING and IFN-β in peri-implant tissues on day 7 with different treatments by immunohistochemical staining. (J to M) Representative flow cytometry plots of mature DCs (CD80+CD86+) by gating on CD11c+ MHC II+ cells, activated neutrophils (CD54+CD182+) by gating on CD11b+Ly6G+ cells, CD4+ T cells (CD4+) by gating on CD3+ T cells, and plasmablasts (CD138+CD19+) by gating on CD45+ cells. DCs and neutrophils were analyzed from locally infected tissues on day 7. T and B cells were analyzed from IDLNs on day 7. (N) Quantitative analysis of the corresponding immune cell population. (O) IgM and (P) IgG antibody levels in the serum of mice on day 7 from different treatment groups. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns means no significance).