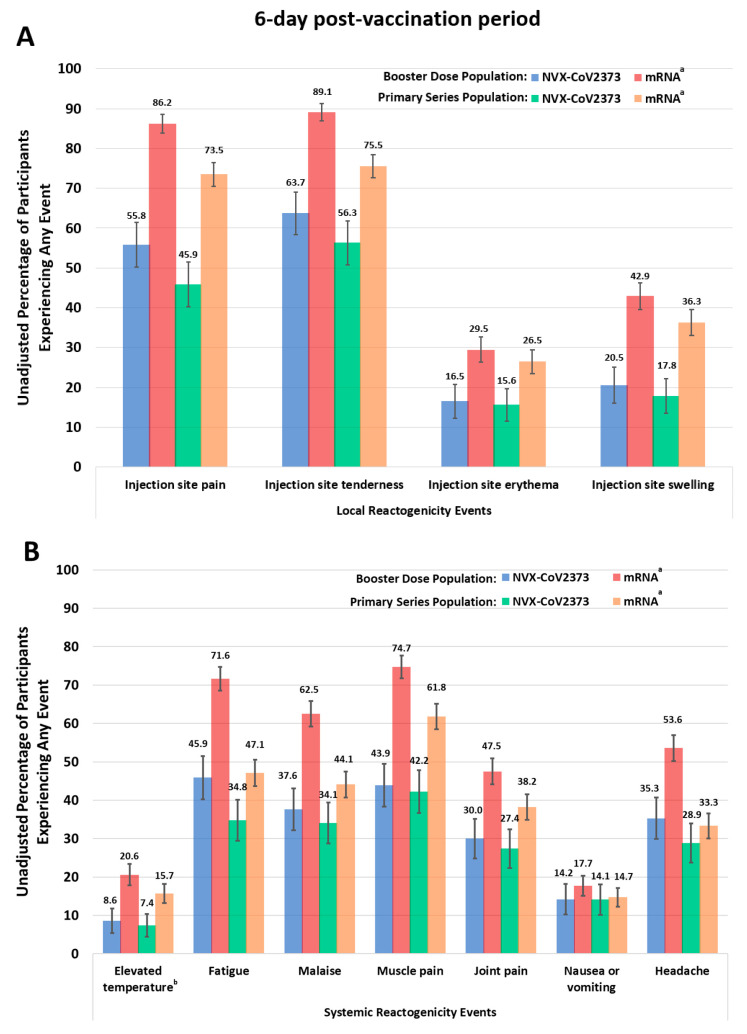
Figure 3.
Responses to the Vaccine Symptoms Diary ((A), local, and (B), systemic reactogenicity) in the 6-day post-vaccination period for either the booster or primary series doses. Participants experienced an event if a grade 1 or worse reactogenicity event was reported for any day during the 6-day post-vaccination period. a Individuals received either BNT162b2 or mRNA-1273. b Temperatures at or above 100.4 °F were considered elevated.