Abstract
The purpose of this study was to evaluate the tissue response to a 70% polyhydroxybutyrate and 30% hydroxyapatite composite in the form of a bone implant, placed intracortically in the distal metaphyseal of the right femur, and subcutaneous implants in cats. Samples of the composite were implanted subcutaneously in the dorsolumbar region and the distal metaphyseal region of the right femur of the animals. The study used 12 neutered adult mixed breed cats, weighing an average of 3.5 kg. The cats were randomly divided into three groups: GI, GII and GIII, according to the length of the assessment period. The assessments of their subcutaneous and bone tissues were performed at 15, 30 and 45 days and at 30, 60 and 90 days, respectively. The subcutaneous and bone reactions to the composites were characterized by granulomatous inflammation with a predominance of macrophages and giant cells. The results showed that the composites triggered a chronic local inflammatory response, despite their clinical acceptance.
The population of domestic cats has grown in recent years to the point that it has surpassed the dog population in the United States, China and many European countries. 1 Therefore, it is expected that there will be an increase in the number of traumatic injuries, such as long bone fractures, seen in these animals. Femoral fractures occur with an average frequency of 20.8%. 2 The primary causes of fractures in cats include car accidents and falls from heights. 3 The technological advances in the field of biomaterials have promoted the production of resorbable implants, which precludes the need for a second procedure to remove the hardware. 4,5 The use of this type of material is important in veterinary medicine when working with animals that should not be excessively handled, such as some cats, birds and wild animals. The handling of these animals usually results in stress and interferes in the recovery process; thus, it should be kept to a minimum. Furthermore, the biomaterial should promote the regeneration of adjacent tissue and cause minimal inflammatory reaction. However, there are only a few biomaterials that meet these requirements, and their use is not feasible due to high cost. 4,5 Thus, there is a relentless quest to find materials that are efficient in regards to both organic performance and affordability.
The polyhydroxybutyrate/hydroxyapatite (PHB/HA) composite emerges as an alternative to the production of resorbable orthopedic implants. 6–10 This composite has both chemical and mechanical properties that are favorable for use as bone implants. 10 Its physical, chemical and structural properties are similar to the structure and chemical composition of bone. In addition, it is biodegradable and allows for the resorption of the polymer over time, and therefore, allows healing of the fracture. 6,9 The use of this product in the form of an orthopedic implant may be advantageous because, in addition to stabilization, there would be a gradual loss of physical resistance, which favors the process of new bone formation. 8,11
The physical and chemical characteristics of the PHB/HA composite, and the lack of a biological assessment of this product, led to the development of the present study, which aims to assess 70% PHB and 30% HA composite implants in cats for future use in the production of orthopedic implants for cats, dogs and wild animals.
Materials and methods
This study was approved by the Animal Ethics Committee (CETEA/UFMG) under protocol 125/07.
The implants were produced from plates consisting of a 70% PHB (Biocycle; PHB Industrial, São Paulo, Brazil) and 30% HA (HAP-91; JHS Laboratório Químico S/A, Minas Gerais, Brazil) composite. The plates were rectangular, 85 mm long×10 mm wide×3 mm thick, and were produced at the JHS laboratory (Químico S/A, Minas Gerais, Brazil). The plates were sterilized with ethylene oxide; they were then analyzed by X-ray diffraction and scanning electron microscopy associated with energy-dispersive X-ray spectroscopy, to assess the structural characteristics of the composite.
With the animals placed in the sternal recumbency position, the dorsolumbar region was prepared for aseptic surgery. A cutaneous incision was made parallel to and about 3.5 cm from the dorsal midline from the third to fifth lumbar vertebrae. The subcutaneous tissue was dissected with a hemostat, and a 10×10 mm plate fragment was implanted. The skin was then sutured closed with 4/0 nylon (Mononylon; Ethicon, São Paulo, Brazil). The same procedure was performed on the contralateral side. After aseptic preparation of the right hindlimb, the femur 11 was accessed to expose the distal metaphyseal region. The periosteum was sectioned longitudinally and its margins were separated. An aperture was drilled with a 4 mm drill bit using a pneumatic drill (Pneumatic drill; 3M, Brazil) with a controllable speed and constant irrigation with saline solution. A manual trephine with a 4 mm internal diameter was used to remove a cylindrical piece of the composite plate, which was introduced into the bone aperture using finger pressure to level the implant with the bone surface. The periosteum was repositioned over the implanted material, the fascia lata was sutured with simple interrupted sutures and the elimination of dead space was performed with simple continuous sutures; 3/0 absorbable sutures (Polyglycolic acid; Brasuture Indústria Comércio Importação e Exportação, Brazil) were used for both. Skin sutures were placed with 4/0 nylon (Mononylon; Ethicon). After the surgery, the animals were randomly divided into three groups (GI, GII and GIII), with four animals in each group for clinical, radiographic and histological assessment. During the postoperative period, 0.2 mg/kg meloxicam (Maxicam; Ouro Fino Saúde Animal, São Paulo, Brazil) was administered orally every 24 h for three consecutive days.
The surgical wound was evaluated daily for the presence of inflammation. Lameness and pain sensitivity were also assessed. The distal femur was radiographed in the craniocaudal and mediolateral positions before surgery, immediately after and at 30, 60 and 90 days postoperatively. All 12 animals were evaluated at 30 days, eight animals were evaluated at 60 days and four at 90 days. In the mediolateral projection, the implant and the adjacent area were divided into four quadrants, each representing 25% of the analyzed area. 12 In the craniocaudal projection, the area corresponding to the implant and the surrounding area were also divided into four parts, each representing 25% of the analyzed area. 12 The bone–implant interface and the degree of radiopacity of the adjacent tissue were evaluated with the aid of a magnifying lens. For the evaluation of the bone–implant interface, the presence of radiolucency in any of the quadrants would nullify the quadrant, allowing the consideration of only the remaining quadrants for the determination of the bone–implant interface density level. 12 The bone density of the tissue adjacent to the implant was also evaluated. For this evaluation, the sum of the areas that showed an increase in radiopacity was considered. 12
The histological assessments of biopsy samples of subcutaneous tissue were performed at 15, 30 and 45 days and for bone tissue were performed at 30, 60 and 90 days, corresponding to groups GI, GII and GIII, respectively. At the end of each evaluation period, the animals were anesthetized again and subjected to a biopsy for removal of the implants and adjacent tissue. The tissue surrounding the implant was sectioned and the implants were removed. At this time, the presence of fluid, its color and texture were assessed, as well as the appearance of the tissue adjacent to the implant. When fluid was present, samples were collected and sent to the laboratory for bacterial culture. The samples were inoculated in blood agar and cultivated according to the protocol suggested by Quinn. 13 A bone biopsy was performed using a trephine drill with an 8 mm internal diameter connected to a pneumatic drill (Pneumatic Drill; 3M, Brazil) with a controllable speed and constant irrigation with saline solution.
The bone defect derived from biopsy was filled with synthetic hydroxylapatite. All the animals were clinically and radiographically monitored until full recovery and then given up for adoption. In postoperative, meloxicam 0.2 mg/kg was orally administered each 24 h during three consecutive days. The samples obtained were fixed in 10% buffered formalin for 15 days, decalcified for 21 days in 10% formic acid that was buffered with sodium citrate to pH 4.5 and washed in running water.
The bone implants were removed to facilitate cutting with steel blades. They were then routinely processed for paraffin embedding. Sections, each 4 μm thick that included the tissue–implant interface area, were obtained using a microtome and mounted on glass slides. Two slides were obtained from each block; they were stained using the hematoxylin and eosin technique and analyzed by light microscopy. The degree of tissue reaction was evaluated, taking into consideration the presence of connective tissue, inflammatory cells, necrosis and the presence or absence of bone growth areas at the bone–implant interface.
A completely randomized experimental design was used, and the clinical, surgical, radiographic and histological analyses were evaluated descriptively.
Results
Scanning electron microscopy of the composite samples revealed a relatively uniform distribution of particles in the polymer matrix, with sizes ranging from 1 to 20 μm (Fig 1). The energy-dispersive X-ray analysis indicated that the particles consisted of calcium and phosphorus. The composition of the particles analyzed by X-ray diffraction characterized them as HA.
Fig 1.
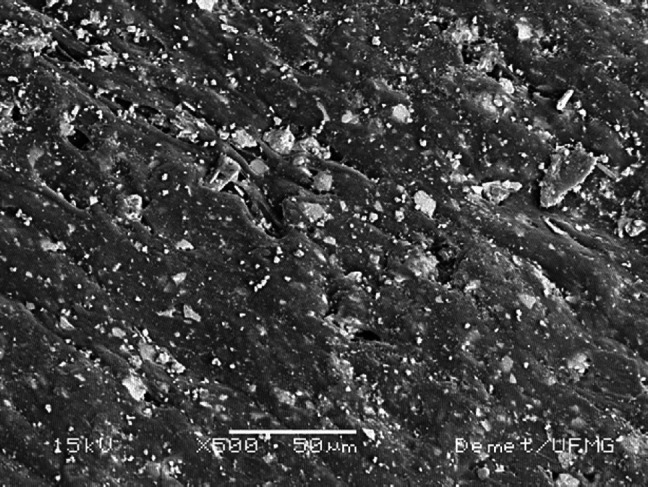
Micrograph obtained by scanning electron microscopy from the PHB/HA composite sample.
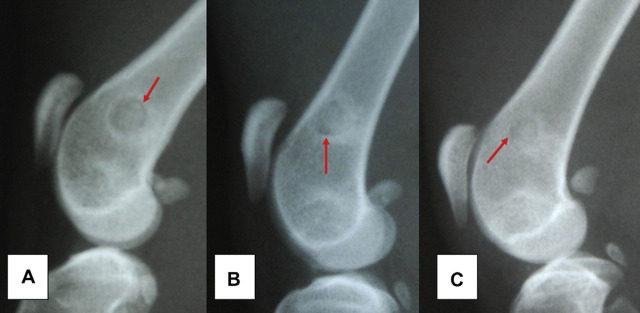
The animals adapted well to the catteries and did not show any clinical complications that could compromise the results of the study. Ambulation occurred immediately after recovering from the anesthesia without lameness. The postoperative radiographic examinations allowed for the verification of the degree of bone density at the bone–implant interface and in the adjacent bone tissue at the different time periods studied. The radiographic images immediately after surgery showed areas of radiolucency around the implants in eight animals (66.67%) and in the medullary region adjacent to the implant in five (41.67%). This radiolucency was reduced at the subsequent assessment periods, but it remained present until the end of the study in three animals from group GIII (75%) (Fig 2). All subcutaneous implants were covered by a fine fibrous capsule. After opening the capsule for the removal of the implants, an odorless brownish mucous secretion was observed surrounding the implant. This secretion was observed in 16 cases (66.67%): four (25%) from group GI (15 days), four (25%) from group GII (30 days) and eight (50%) from group GIII (45 days). The culture results of the fluid were negative in all cases. The composite samples from the subcutaneous tissue showed the same shape and consistency as prior to implantation.
Fig 2.
Radiography of the distal femoral region of cats where it was implanted 70% PHB and a 30% HA composite. Note the reduction of radiolucent line around the implant over time assessment (red arrow). The images identified by the letters (A), (B) and (C) correspond to the time of evaluation 30, 60 and 90 days, respectively.
Microscopic analysis of the capsule and the tissue adjacent to the implant in the subcutaneous tissue revealed a granulomatous inflammatory response in all samples. The formation of a vascularized fibrous capsule around the implant and an inflammatory response was observed in all cases (Fig 3). Focal areas of necrosis were observed in the four animals (100%) from group GI (15 days). Among the collagen fibers and fibroblasts, there was a mixed inflammatory infiltrate that was mainly characterized by the presence of epithelioid macrophages, plasmocytes and lymphocytes (Fig 3). The presence of neutrophils was more evident in animals from group GI. A large number of macrophages and multinucleated giant cells were observed in all periods studied, and some had vacuoles in their cytoplasm containing a birefringent amorphous substance that was similar in appearance to the composite material.
Fig 3.
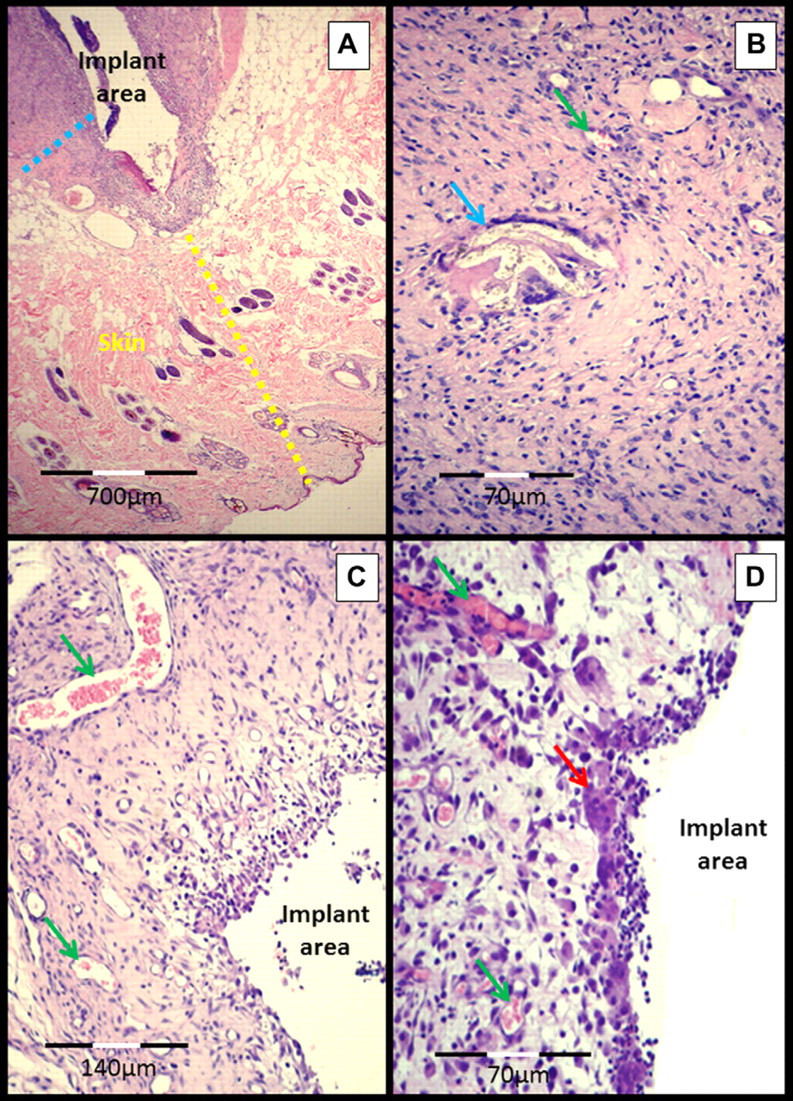
Micrograph of the subcutaneous–implant interface 45 days after experimental surgery for the implantation of a 70% PHB and a 30% HA composite in the subcutaneous tissue of cats. (A) Skin (yellow dotted line), subcutaneous tissue and fibrous capsule over the implant (blue dotted line). (B) Fibrous capsule in greater detail, showing the biomaterial (birefringent appearance) inside a giant cell (blue arrow), the green arrow points to a vessel. (C) Fibrous capsule with intense neovascularization (green arrows) and multinucleated giant cells (red arrow).
Macroscopic evaluation of the bone showed the implants firmly adhered and covered by the periosteum. None of the animals that were subjected to biopsy at 30, 60 and 90 days showed macroscopic signs of degradation of the implant, such as changes in shape, consistency or texture. Two animals (50%) from group GIII (90 days) had implants covered by a thin layer of dense whitish tissue, which was microscopically characterized as fibrous connective tissue.
Histological evaluation enabled the analysis and characterization of the different cell types at the bone–implant interface. There was a similar tissue response for all of the evaluated groups (GI, GII and GIII). The bone–implant interface consisted of fibrous connective tissue in all bone samples, and there was a mononuclear inflammatory infiltrate, of mild to moderate intensity, mainly characterized by the presence of macrophages, lymphocytes and multinucleated giant cells around the implant at all of the assessment times (Fig 4). Focal areas of bone necrosis were observed in the tissue adjacent to the implant. There was no new bone formation at the areas in direct contact with the composite. The presence of macrophages and multinucleated giant cells with a cytoplasm full of vacuoles and containing a birefringent amorphous substance similar to the composite was evident in all of the groups studied.
Fig 4.
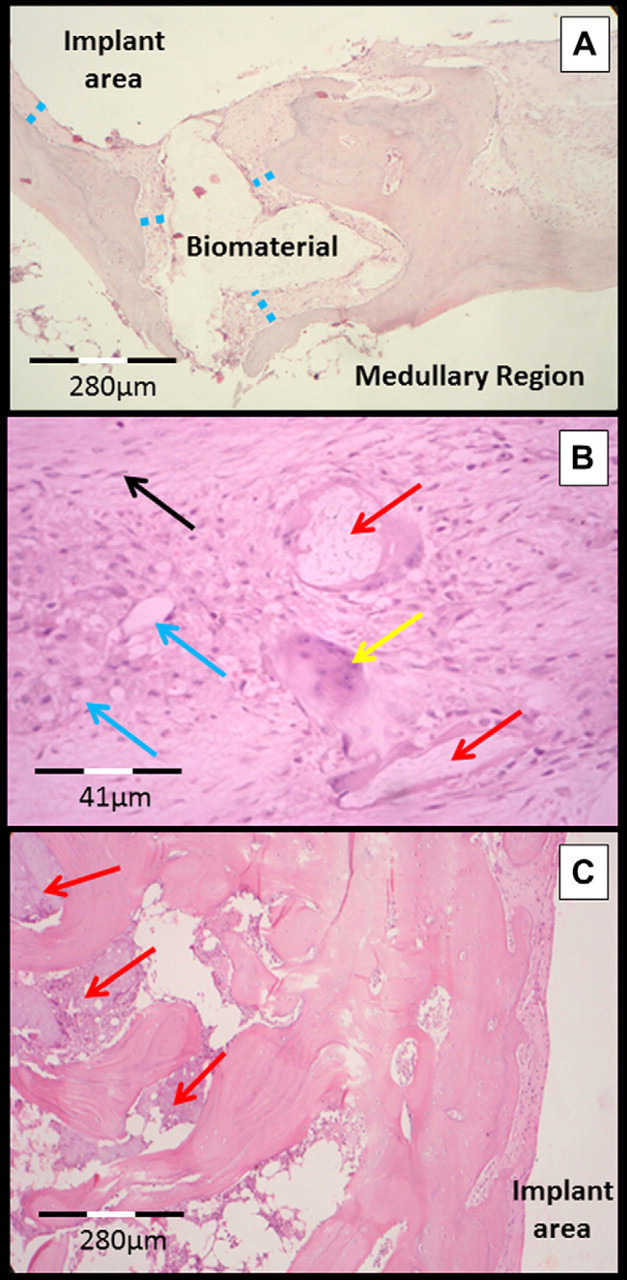
Micrograph of the bone–implant interface in the distal femoral metaphysis of cats. (A) Biomaterial (birefringent appearance) surrounded by connective tissue (blue dotted line) and by bone tissue (assessment at 60 days). (B) Fibrous capsule in greater detail showing the inflammatory infiltrate mainly composed of macrophages (blue arrows) and multinucleated giant cells (yellow arrows). The red arrow points to biomaterial (birefringent appearance) inside a giant cell and the black arrow indicates a fibroblast (assessment at 60 days). (C) Focal area of bone necrosis close to the area of implantation of biomaterial (red arrows) (assessment at 90 days).
Discussion
One of the ways to evaluate PHB/HA composites as a biomaterial is to observe the response when they are implanted into tissue. This information is essential when the clinical use of a material is predicted. 14 The distal third of the femur was elected as an experimental model in this study due to its osteogenic properties 12,14 and its anatomical conformation, which provides easy surgical access and, because it is the widest region of the femur, enables the placement and extraction of an 8 mm diameter bone–implant section with minimal weakening of the bone. The lumbar region of the spine is an area easily accessed and favors minimal tissue injury, 15 which leads to minimal formation of fibrous tissue in the implant area, thus decreasing the risk of a reaction stemming from the surgical trauma.
The negative result of the tissue and fluid cultures suggests that the observed response is an aseptic inflammatory reaction. The surgical intervention and presence of an implant are, in themselves, an assault on the body and induce an inflammatory response, which is necessary for tissue repair. The microstructural and topographic composition of the surface of the biomaterial also influences the biological response. 14,16
As in this study, the formation of a fibrous capsule and the presence of a macrophage infiltrate with giant cells have also been reported at 45 days after subcutaneous implantation of a PHB/HA composite in rats. 9 The resorption of only PHB was reported at 30 days after implantation in the subcutaneous tissue of rats, with a reduction of 9.47% in the mass of the implant. 17 In this study, signs of resorption, such as macrophages and multinucleated giant cells containing vacuoles with birefringent substances, were observed after 15 days of implantation, and the subcutaneous tissue response may have been enhanced by the movement in the area 14 because it was observed in all study groups. The tendency for the inflammatory infiltrate to decrease and replacement by a predominance of mononucleated cells suggest a chronicity to the inflammatory process over time. 17 No new bone formation in areas adjacent to subcutaneous implants has been reported in rats 9 or rabbits. 6
Radiolucent areas located in the medullary region adjacent to the implants were attributed to the removal of bone marrow fragments when the defect was created, as has been reported in other studies. 12,15 However, the radiolucent areas observed at 30, 60 and 90 days could be associated with the presence of connective tissue in the bone–implant interface.
The focal areas of bone necrosis observed surrounding the implant in this study are probably associated with granulomatous inflammation (Fig 3). Nevertheless, the drilling of the orifice may heat the drill bit and consequently heat the bone, which may lead to cell death. Other studies performed in rabbits on materials with a similar composition showed an osteoconductive effect of the composite with the formation of lamellar bone on the bone–implant interface. 6,8 The bone response to both components of the PHB/HA composite has been studied, but the results are still controversial. 4,18–20 Some studies report an osteoconductive effect of HA with colonization of its surface by osteoblasts and growth of lamellar bone. 4,20 However, there are other studies that report negative effects of HA, such as an exacerbated inflammatory response 18 or even implant rejection. 19 It is believed that the biological efficiency of HA is associated with several factors such as its origin, purity and implant design. 14 The response seen in this study could be due to the low amount of HA, the osteoconductive component of the composite. 20 PHB, the other component of the composite used in this study, is a polymer known to be biocompatible, resorbable, and known for its mechanical properties that are favorable for the production of orthopedic implants, 17 especially when reinforced with HA. 6 –9 However, PHB stimulates a chronic inflammatory response for periods exceeding 12 months, generally producing encapsulation and gradual resorption of the implant, 5,6,17 which justifies the results found in this study. The purpose of using this material in this study was for slow resorption, which is why it has a high density.
The presence of macrophages and giant cells with a cytoplasm full of vacuoles containing a birefringent amorphous substance similar to the composite was evident in all of the groups studied and suggests resorption of the composite at 30 days after bone implantation. This is in contrast to a similar study that did not find evidence of resorption of the PHB/HA composite earlier than 3 months after implantation in the femurs of rabbits. 6
An evident inflammatory response to the PHB/HA composite was observed in the present work. This may impair the repairing of the adjacent tissues and lead to rapid reabsorption of the implant, what would consequently cause its mechanical failure. As much as the inflammation, the implant mechanical failure can delay or even hinder the fracture consolidation, what may take to lameness, angular deviations or, even, bone non-union.
Conclusion
Given the conditions in which this study was performed, and considering the results gathered, it may be concluded that: the PHB (70%) and HA (30%) composite triggers a chronic local inflammatory response and must be further studied before it is used as an orthopedic implant in animals.
Acknowledgements
The authors are grateful to CAPES for the Masters Scholarship, the School of Veterinary Medicine and the Research Provost Office from the Universidade Federal de Minas Gerais for the support in completing this work.
References
- 1. Bernstein P. The human–cat relationship. Rochlitz I. The welfare of cats, 2005, Springer: Dordrecht (The Netherlands), 47–89. [Google Scholar]
- 2. Nolte D.M., Fusco J.V., Peterson M.E. Incidence of and predisposing factors for nonunion of fractures involving the appendicular skeleton in cats: 18 cases (1998–2002), J Am Vet Med Assoc 226, 2005, 77–82. [DOI] [PubMed] [Google Scholar]
- 3. Worth A.J. Management of fractures of the long bones of eight cats using external skeletal fixation and a tied-in intra-medullary pin with a resin-acrylic bar, N Z Vet J 55, 2007, 191–197. [DOI] [PubMed] [Google Scholar]
- 4. De Long W.G., Jr., Einhorn T.A., Koval K., et al. Bone grafts and bone substitutes in orthopaedic trauma surgery. A critical analysis, J Bone Joint Surg Am 89, 2007, 649–658. [DOI] [PubMed] [Google Scholar]
- 5. Rezwan K., Chen Q.Z., Blaker J.J., Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering, Biomaterials 27, 2006, 3413–3431. [DOI] [PubMed] [Google Scholar]
- 6. Doyle C., Tanner E.T., Bonfield W. In vitro and in vivo evaluation of polyhydroxybutyrate and polyhydroxybutyrate reinforced with hydroxyapatite, Biomaterials 12, 1991, 841–847. [DOI] [PubMed] [Google Scholar]
- 7. Knowles J.C., Hastings G.W., Ohta H., Niwa S., Boeree N. Development of a degradable composite for orthopaedic use: in vivo biomechanical and histological evaluation of two bioactive degradable composites based on the polyhydroxybutyrate polymer, Biomaterials 13, 1992, 491–496. [DOI] [PubMed] [Google Scholar]
- 8. Luklinska Z.B., Schluckwerder H. In vivo response to HA-polyhydroxybutyrate/polyhydroxyvalerate composite, J Microsc 211, 2003, 121–129. [DOI] [PubMed] [Google Scholar]
- 9. Shishatskaya E.I., Khlusov I.A., Volova T.G. A hybrid PHB-hydroxyapatite composite for biomedical application: production, in vitro and in vivo investigation, J Biomater Sci Polym Ed 17, 2006, 481–498. [DOI] [PubMed] [Google Scholar]
- 10. Alves E.G.L., Rezende C.M.F., Oliveira H.P., Borges N.F., Mantovani P.F., Lara J.S. Avaliação mecânica da placa de compósito de poli-hidroxibutirato e hidroxiapatita em modelos ósseos de gato, Arq Bras Med Vet Zootec 62, 2010, 1367–1374. [Google Scholar]
- 11. Piermattei D.L., Johnson K.A. Atlas of surgical approaches to the bones and joints of the dog and cat, 2004, Saunders: Philadelphia, 400. [Google Scholar]
- 12. Sá M.J., Rezende C.M., Junior V.A. Silva, Garcia H.C., Griffon D.J., Silva V.V. In vivo behavior of zirconia-hydroxyapatite (ZH) ceramic implants in dogs: clinical, radiographic, and histological study, J Biomater Appl 22, 2007, 5–31. [DOI] [PubMed] [Google Scholar]
- 13. Quinn P.J., Markey B.K., Carter M.E., Donnelly W.J., Leonard F.C. Veterinary microbiology and microbial diseases, 2002, Blackwell: Iowa, 536. [Google Scholar]
- 14. Rezende C.M.F. Teste in vivo de biomateriais e histotécnicas. Oréfice R.L., Pereira M.M., Mansur H.S. Biomateriais fundamentos e aplicações, 2006, Cultura Médical: Rio de Janeiro, 299–314. [Google Scholar]
- 15. Manjubala I., Sivakumar M., Sureshkumar R.V., Sastry T.P. Bioactivity and osseointegration study of calcium phosphate ceramic of different chemical composition, J Biomed Mater Res 63, 2002, 200–208. [DOI] [PubMed] [Google Scholar]
- 16. Jones J.R., Hench L.L. Regeneration of trabecular bone using porous ceramics, Curr Opin Solid State Mater Sci 7, 2003, 301–307. [Google Scholar]
- 17. Gogolewski S., Jovanovic M., Perren S.M., Dillon J.G., Hughes M.K. Tissue response in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxibutirate) (PHB), and poly(3-hydroxibutyrate-co-3-hydroxivalerate) (PHB/VA), J Biomed Mater Res 27, 1993, 1135–1148. [DOI] [PubMed] [Google Scholar]
- 18. Van Blitterswijk C.A., Grote J.J., Kuypers W., Blok-van Hoek C.J., Daems W.T. Bioreactions at the tissue/hydroxyapatite interface, Biomaterials 6, 1985, 243–251. [DOI] [PubMed] [Google Scholar]
- 19. Oguchi H., Ishikawa K., Mizoue K., Seto K., Eguchi G. Long-term histological evaluation of hydroxyapatite ceramic in humans, Biomaterials 16, 1995, 33–38. [DOI] [PubMed] [Google Scholar]
- 20. Mygind T., Stiehler M., Baatrup A., et al. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds, Biomaterials 28, 2007, 1036–1047. [DOI] [PubMed] [Google Scholar]