A 5-year-old cat was presented with a history of episodic weakness and cervical ventroflexion. Screening blood tests revealed moderate hypokalaemia, mild hypernatraemia, and grossly elevated creatine kinase (CK) levels. On clinical examination, a large mass was palpable adjacent to the left kidney. Ultrasonographic examination confirmed this mass to be an enlarged left adrenal gland, a finding which in association with the history and laboratory data was suggestive of primary aldosteronism (Conn's syndrome) due to unilateral adrenal neoplasia. The diagnosis was confirmed by demonstrating a markedly elevated circulating aldosterone concentration. The cat was initially treated supportively and then underwent unilateral adrenalectomy. Histopathology of the adrenal gland confirmed the presence of an adrenocortical adenoma. Post-operatively the cat recovered well and had no known further problems (follow-up period currently 20 months). This is the first published case of successful surgical treatment of aldosteronism in the cat.
Primary aldosteronism was first described in humans by Conn in 1955 and is consequently known as ‘Conn's syndrome’. In humans, the disease occurs as a result of uncontrolled overproduction of aldosterone by the zona glomerulosa of the adrenal gland, usually due to either unilateral neoplasia (adenoma or, rarely, adenocarcinoma) or bilateral idiopathic hyperplasia. The resultant aldosterone excess can lead to a variety of disorders including electrolyte imbalances (hypokalaemia, hypernatraemia), hypertension, and metabolic alkalosis (Young 1997). The classic clinical manifestation of the disease is muscle weakness due to hypokalaemia. The definitive diagnosis of primary aldosteronism is based upon the detection of inappropriately elevated serum aldosterone levels (Young 1997). Measurements of plasma renin activity are typically subnormal in these patients due to negative feedback stimulus from the elevated aldosterone concentration.
Primary aldosteronism has been infrequently reported in the cat. To the authors' knowledge, only a single case of this disease in the cat has been reported (Eger et al 1983). This 17-year-old cat displayed chronic intermittent weakness and depression and had profound hypokalaemia detected on blood sampling. Serum aldosterone levels were markedly elevated, and plasma renin activity was marginally subnormal. Clinical signs were successfully controlled with potassium supplementation and spironolactone (an aldosterone antagonist) for 2.5 months, but the cat subsequently developed renal failure and was euthanased. A post-mortem examination confirmed the presence of an adrenocortical adenocarcinoma. This report describes a case of feline primary aldosteronism that was cured via unilateral adrenalectomy.
Case history
A 5-year-old neutered male domestic longhaired cat was referred to The Feline Centre at the University of Bristol. He had been presented to the referring veterinary surgery four times in the previous year for investigation of severe episodic weakness and possible ataxia. On the first two occasions, the clinical signs had resolved by the time the cat had been presented to the referring veterinary surgeon for examination. No abnormalities were detected on routine clinical examinations and no diagnostic tests were performed. The cat was treated empirically with amoxycillin (Amoxypen; Intervet UK) and dexamethasone (Dexafort; Intervet UK).
The owners of the cat videotaped part of the third episode, and showed this to the veterinary surgeon on their third visit. While the cat was again asymptomatic on presentation, the videotape demonstrated the cat displaying marked muscle weakness characterised by cervical ventroflexion, inability to raise the head, and apparent hindlimb ataxia. The veterinary surgeon performed routine haematology and serum biochemistry (not including an electrolyte assessment) which were unremarkable. The cat was given the same treatment as previously.
On the fourth occasion, the cat was presented to the veterinary practice still in a collapsed state. A clinical examination performed by the veterinary surgeon revealed tachypnoea, pale mucous membranes, and a Grade II/VI systolic murmur. Repeat in-house haematology revealed anaemia (packed cell volume [PCV] 15%, reference range 25–45%) with apparent erythrocyte agglutination. A serum biochemistry panel was again unremarkable, including thyroxine (T4) measurement. Tests for feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) were negative, and samples sent for Coombs' testing and antinuclear antigen (ANA) were also negative. The cat was given no treatment but improved within the following 24 h, although he remained quiet and depressed.
Further blood samples were taken and submitted to the University of Bristol's Clinical Pathology Diagnostic Service. Haematology at that point revealed a mild anaemia (PCV 22.4%) with evidence of active regeneration. In addition, some agglutination and rouleaux were detected. The total white cell count was normal (11.0 × 109/l, reference range 5.5–22.0 × 109/l), although a small number (0.22 × 109/l) of unidentified blast cells were present in the circulation. No Haemobartonella felis was detected, and a repeat Coombs' test was negative at 4°C and 37°C. An extensive serum biochemistry panel revealed a mild increase in total proteins (81.2 g/l, reference range 54–78 g/l) and ALT (82 U/l, reference range <70 U/l). More significantly, a moderate hypokalaemia (3.4 mmol/l, reference range 4.0–5.0 mmol/l) and marked elevation in creatine kinase (37 556 U/l, reference range <150 U/l) were present. A mild hypernatraemia was also detected (161.5 mmol/l, reference range 145–160 mmol/l). Urea and creatinine levels were within normal range. After obtaining these results, the cat was referred to the University of Bristol's Feline Centre for further evaluation.
On presentation at the Feline Centre, the cat was quiet but responsive to stimuli and weighed 4.5 kg. He was thin and had a dull hair coat. The cat was observed to walk around the room, with no evidence of muscle weakness, cervical ventroflexion, or ataxia. A neurological examination was unremarkable. Physical examination revealed a Grade II/VI systolic heart murmur with a point of maximal intensity over the left hemithorax and a palpable abdominal mass located cranial and medial to the left kidney. The remainder of the examination was unremarkable.
The cat was admitted for further investigations. Haematological results were similar to the previous findings, with a mild regenerative anaemia (PCV 21.1%), erythrocyte agglutination/rouleaux, and a normal white blood cell count. However, no abnormal leukocytes were detected, and a bone marrow aspirate revealed active erythrocyte regeneration but was otherwise unremarkable. On serum biochemistry, the potassium had dropped to 2.9 mmol/l, and the creatine kinase had increased to 47 335 U/l. The ALT was again mildly elevated (111 U/l). Total protein and sodium levels were normal.
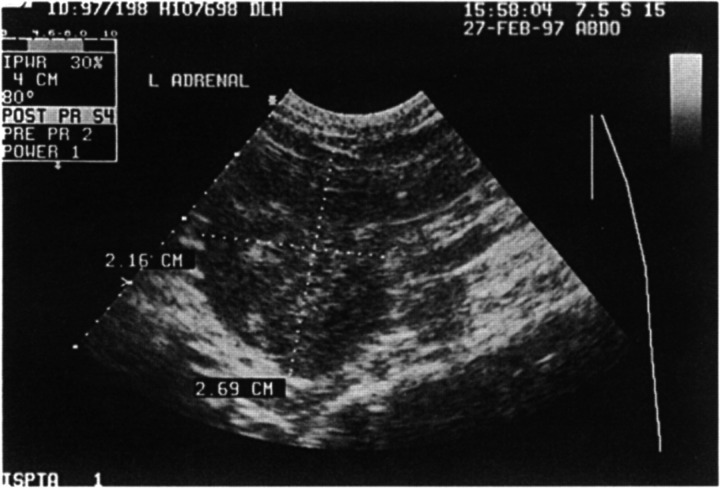
Electrocardiography, thoracic radiography, and echocardiography were all unremarkable, and therefore the heart murmur was thought to be haemic in origin. Abdominal radiography revealed an irregular area of increased radio-density cranial to the left kidney, corresponding to the palpable mass. Ultrasonographic examination of this area revealed a markedly enlarged left adrenal gland of heterogeneous echogenicity, but there was no evidence of invasion of the caudal vena cava (Fig 1).
Fig 1.
Ultrasonographic appearance of the enlarged left adrenal gland.
A diagnosis of primary aldosteronism was suspected based on these findings, and the diagnosis was subsequently confirmed by detecting a markedly elevated plasma aldosterone concentration (PAC) of 39 000 pmol/l (reference range for 14 healthy cats 150–430 pmol/l). However, this result was obtained after surgical treatment of the cat.
An adrenocorticotrophic hormone (ACTH) stimulation test performed to assess glucocorticoid activity was normal [with cortisol results before, and both 1 and 3 h after an intravenous injection of 0.125 mg tetracosactrin (Synacthen; Ciba Laboratories) being <20 nmol/l, 110 mmol/l and 106 mmol/l respectively]. Indirect (Doppler) systolic blood pressure measurements were normal on two separate occasions (130–140 mmHg) and retinal examination revealed no evidence of hypertensive changes.
The cat was initially treated with potassium supplementation, intravenously (30 mmol KCl/l in Hartmanns fluid at maintenance rate for 12 h) and then orally (Tumil-K [potassium gluconate]; Daniels Pharmaceuticals, 4 mmol po twice daily). Blood potassium concentration gradually increased to 3.7 mmol/l and the cat appeared brighter clinically.
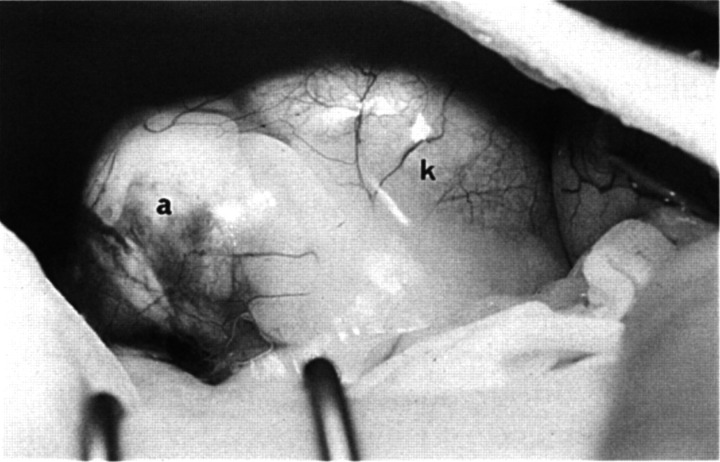
Immediately prior to surgery for adrenalectomy which was undertaken 7 days after admission to the hospital, the cat was given a slow intravenous infusion of hydrocortisone (0.5 mg/kg/h), which was considered prudent as the precise nature of the adrenal mass had not been confirmed at that stage. However, in view of the normal results from the ACTH stimulation test, in retrospect, this was probably unnecessary (see below). A mid-line laparotomy was performed to allow access to, and ideally visualisation of, both adrenal glands. The enlarged left adrenal gland (3.5×3×2.5 cm) was identified at the cranial pole of the left kidney (Fig 2). It was removed in total, and although the right adrenal gland could not be visualised, it was palpable and appeared to be of normal size and texture. Histopathological examination of the left adrenal gland revealed a lobulated, well-encapsulated adrenocortical adenoma.
Fig 2.
Adrenal mass (a) adjacent to left kidney (k) at exploratory laparotomy.
Post-operatively the hydrocortisone infusion was gradually tapered and discontinued over a 24-h period. Blood potassium concentration was checked daily; normokalaemia was achieved within 5 days and all subsequent potassium measurements were within normal range. The CK value declined steadily, reaching a normal level of 114 U/l, 6 days postoperatively. The cat also showed good clinical improvement.
Eight days following surgery, a repeat haematology revealed a PCV of only 16% (down from the pre-operative value of 23%). Regenerative characteristics were again present. Repeat tests for Coombs antigen and Haemobartonella felis were negative, but abdominal ultrasonography revealed a small amount of free fluid (thought to be blood) in the abdomen and also pocketed at the adrenalectomy site.
The cat recovered fully from surgery and was discharged. Follow-up examinations were unremarkable, with periodic blood tests throughout the past 20 months revealing no further evidence of hypokalaemia, hypernatraemia, or CK elevation. The anaemia also resolved, with the PCV reported to be normal by the referring veterinary surgeon 4 weeks after the cat's discharge from the University of Bristol. The cat has had no further significant medical problems and is currently doing well, 20 months after surgery.
Discussion
Aldosterone is a potent mineralocorticoid with two primary functions: to prevent hyperkalaemia and to prevent hypotension. Normally its production and release are stimulated through the renin-angiotensin-aldosterone axis by a complexity of renal chemoreceptors and baroreceptors that detect hyperkalaemia, hyponatraemia, and renal hypotension. The multiple effects of aldosterone occur at the renal cellular level, influencing the activity of sodium/potassium pumps and membrane channels. Sodium reabsorption and potassium excretion are increased, thus restoring electrolyte balance. Sodium retention also induces relative hypervolaemia and consequently raises renal arteriolar blood pressure, combating hypotension (Ahn 1994).
In primary aldosteronism, neoplastic or hyperplastic cells in the zona glomerulosa secrete aldosterone autonomously, leading to markedly raised circulating levels. Sodium is thus retained excessively, which may result in systemic hypertension, and severe kaliuresis occurs. In addition, negative feedback stimulation from the high aldosterone concentration on the juxtaglomerular apparatus results in decreased renin release.
In this and the previous case of feline primary aldosteronism, presenting clinical signs were due to hypokalaemia. However, primary aldosteronism is a rare cause of hypokalaemia in the cat (Ahn 1994). The most common cause of hypokalaemia in this species is chronic renal failure (Dow et al 1989), with other frequently recognised causes including anorexia, gastrointestinal disease, and hepatic disease (Dow et al 1989, Ahn 1994). In this case, there was no evidence for any of these differentials, increasing the suspicion of primary aldosteronism.
In cats, early potassium deficiency causes hyperpolarisation of muscle fibre membranes, reducing their excitability and thus inducing a state refractory to the transmission of action potentials (Fettman 1989). More chronic and severe hypokalaemia causes muscle fibre hypopolarisation, leading to extreme muscle weakness and eventual exercise-induced rhabdomyolysis, as demonstrated by the detection of elevated CK levels (Fettman 1989). Clinically, the hypokalaemic polymyopathy can be manifested by muscle weakness, stiff gait, myalgia, and/or cervical ventroflexion such as evident in this case (Dow et al 1987). The mild elevation in ALT seen in this case was also thought to be consistent with severe muscle damage (Swenson & Graves 1997).
Hypokalaemia in the cat can also cause various arrhythmias and other electrocardiographic abnormalities, including S-T segment depression, decreased T wave amplitude, and increased QRS amplitude and/or duration (Eger et al 1983). However, no electrocardiographic abnormalities were found in this case despite the cat's hypokalaemia.
A protracted hypokalaemic state can induce kaliopenic nephropathy due to multiple haemodynamic and metabolic changes at the renal level (Fettman 1989). Clinical signs of polyuria/polydipsia and dehydration may result, and blood tests may reveal azotaemia and metabolic alkalosis (Ahn 1994). This syndrome must be distinguished from the more common scenario of hypokalaemia occurring secondarily to primary renal failure. However, in this case, there was no evidence of renal dysfunction.
Hypertension can occur with primary aldosteronism due to the expansion of plasma volume secondary to excessive sodium retention. Although prevalent in human patients, where primary aldosteronism is the most common cause of secondary hypertension (Boon et al 1997), the cat in this report was normotensive and the previously reported cat with primary aldosteronism (Eger et al 1983) did not have blood pressure measurements performed.
Anaemia can potentially occur in primary aldosteronism due to relative erythrocyte mass dilution secondary to hypervolaemia (Monroe 1997). However, the anaemia in this case was both marked and regenerative, and therefore not consistent with this pathogenesis. While operative/post-operative haemorrhage could have accounted for the cat's second reduction in PCV, which was consistent with the abdominal fluid detected by ultrasound post-operatively, the cause of the initial regenerative anaemia remains undetermined in this case despite various tests. There was no direct evidence for preoperative intra-abdominal haemorrhage from the tumour, although this remains a possibility.
An increased PAC with a concurrently decreased plasma renin activity (PRA) due to negative feedback stimulation is suggestive of primary aldosteronism, and characteristic PAC/PRA ratios for this condition have been determined in man (Young 1997). Ideally PAC should not be interpreted singly, as it can also be elevated in secondary hyperaldosteronism, a condition characterised by increased aldosterone secretion as a physiological response to increased plasma renin activity. In this cat, only PAC was measured, and in retrospect PRA would have been useful. However, the extremely elevated PAC implied autonomous secretion, and the presence of an adrenal mass suggested primary adrenal gland neoplasia. In addition, conditions of secondary hyperaldosteronism in the cat are associated with hypovolaemia and/or hyponatraemia and are characterised by the presence of subcutaneous oedema (Ahn 1994); there was no evidence of these abnormalities in this case.
In human patients, the two major subtypes of primary aldosteronism are unilateral aldosterone-producing adenoma (APA) and bilateral idiopathic hyperaldosteronism (IHA) due to hyperplasia of the zonae glomerulosae. There are four other subtypes, including aldosterone-producing adenocarcinomas, but all are uncommon (Young 1997). Imaging modalities can be extremely useful diagnostic adjuncts, as with the ultrasonography in this case. However, the definitive diagnosis of primary aldosteronism in man depends upon demonstrating a lack of PAC suppression in response to sodium chloride loading (Young 1997). This type of test could potentially be adapted to verify cases of presumed primary aldosteronism in the cat.
Initial therapy of primary aldosteronism is aimed at normalising potassium levels. Intravenous fluid therapy with supplemental KCl is indicated (Ahn 1994), especially in cats with marked biochemical hypokalaemia and/or signs of polymyopathy or electrocardiographic abnormalities. Oral potassium gluconate treatment should also be initiated, at a recommended dose of 2 mmol per 4.5 kg body weight po twice daily (Plumb 1995) but adjusted as needed, based on frequent potassium monitoring.
Spironolactone, a synthetically produced homologue of aldosterone, can be useful in the initial treatment of hypokalaemia. It is a competitive antagonist of aldosterone, causing decreased aldosterone binding at the renal cell receptor level (Ahn 1994). Spironolactone was successfully used to control hypokalaemia for 2.5 months in the previously reported cat with primary aldosteronism (Eger et al 1983). This drug is also the treatment of choice for long-term maintenance of human patients with IHA, as surgery is not indicated (Young 1997).
However, unilateral adrenalectomy is recommended in human patients with APAs, and is extremely successful, curing the hypokalaemia in all cases (Young 1997). Clearly adrenalectomy procedures are not without risk, and care must be taken to ensure a stable pre-operative condition and proper anaesthetic monitoring. In this cat's case, corticosteroid supplementation was also initiated prior to surgery, despite an ACTH stimulation assay verifying adequate glucocorticoid activity. In retrospect, this treatment, although harmless, was considered to have been unnecessary for a unilateral adrenalectomy procedure based on human precedent (Young 1997).
Other than the suspected post-operative haemorrhage, there were no recognised surgical or anaesthetic complications in this cat's case. An adrenocortical adenoma was identified on histopathology following complete unilateral adrenalectomy. The surgery was curative, as evidenced by the resolution of all clinical and biochemical abnormalities. The cat remains well, with no recurrence of signs, 20 months postoperatively at the writing of this report.
Feline primary aldosteronism is a rare condition. There are multiple similarities to the disease in man, including characteristic clinical signs and laboratory abnormalities. In addition, many diagnostic methods comparable to those utilised in human medicine can be employed to confirm the presence of this disease in feline patients. In cases of suspected feline primary aldosteronism, clinicians should strive to not only verify the diagnosis but also to identify the probable subtype. If primary unilateral neoplasia (eg aldosterone-producing adenoma) is identified, surgical adrenalectomy may be a curative procedure, as in this case.
Acknowledgements
AD MacKay and AH Sparkes were supported by the Feline Advisory Bureau. The authors wish to thank their colleagues at Bristol Veterinary School who helped with the management of this case.
Addendum
Two years after successful surgery, the cat was re-examined at our clinic for a routine check. Although the cat was clinically normal at this time, a recurrence of the hypokalaemia (3.3 mmol/l) was found on biochemical screening. Further blood was submitted for aldosterone assay, which also revealed recurrence of the hyperaldosteronaemia (14 900 pmol/l). Abdominal radiography revealed a soft tissue mass in the region of the right adrenal gland, and on ultrasonography a lobulated mass could be seen that appeared to be invading the caudal vena cava at this site. Exploratory laparotomy was undertaken via a mid-line approach, which revealed a nodular mass approximately 2 cm × 3 cm at the cranial pole of the right kidney that was heavily infiltrating both the caudal vena cava and surrounding soft tissues. Surgical resection proved impossible, and euthanasia was performed at the owner's request. Subsequent post-mortem examination confirmed the presence of a right adrenocortical carcinoma with local invasion but no evidence of distant metastasis. Although adrenocortical carcinomas are more likely to be bilateral than are adenomas [Capen CC (1995) Endocrine system. In: WW Carlton, MD McGavin (eds) Thomson's Special Veterinary Pathology, 2nd edn. St Louis: Mosby. pp 256–259], this case seems unusual in that a functional carcinoma developed 2 years after the removal of a functional adenoma in the contralateral adrenal gland. However, it suggests that frequent monitoring may be warranted in cats following unilateral adrenalectomy for aldosteronism as bilateral adrenal disease may develop.
References
- Ahn A. (1994) Hyperaldosteronism in cats. Seminars in Veterinary Medicine and Surgery (Small Animal) 9, 153–157. [PubMed] [Google Scholar]
- Boon WC, Coghlan JP, Curnow KM, McDougall JG. (1997) Aldosterone secretion—a molecular perspective. Trends in Endocrinology and Metabolism 8, 346–354. [DOI] [PubMed] [Google Scholar]
- Conn JW. (1955) Presidential address. Part II. Primary aldosteronism, a new clinical syndrome. Journal of Laboratory Clinical Medicine 45, 3–17. [PubMed] [Google Scholar]
- Dow SW, Fettman MJ, Curtis CR, Lecouteur RA. (1989) Hypokalemia in cats: 186 cases (1984–1987). Journal of the American Veterinary Medical Association 194, 1604–1608. [PubMed] [Google Scholar]
- Dow SW, Lecouteur RA, Fettman MJ, Spurgeon TL. (1987) Potassium depletion in cats: Hypokalemic polymyopathy. Journal of the American Veterinary Medical Association 191, 1563–1568. [PubMed] [Google Scholar]
- Eger CE, Robinson WF, Huxtable CRR. (1983) Primary aldosteronism (Conn's syndrome) in a cat; a case report and review of comparative aspects. Journal of Small Animal Practice 24, 293–307. [Google Scholar]
- Fettman MJ. (1989) Feline kaliopenic polymyopathy/nephropathy syndrome. Veterinary Clinics of North America: Small Animal Practice 19, 415–432. [DOI] [PubMed] [Google Scholar]
- Monroe WE. (1997) Diseases of the adrenal gland. In: Practical Small Animal Internal Medicine. Philadelphia, PA: W.B. Saunders Company, p. 1021. [Google Scholar]
- Plumb DC. (1995) Veterinary Drug Handbook, 2nd edition. Ames, IA: Iowa State University Press, p. 563. [Google Scholar]
- Swenson CL, Graves TK. (1997) Absence of liver specificity for canine alanine aminotransferase (ALT). Veterinary Clinical Pathology 26, 26–28. [DOI] [PubMed] [Google Scholar]
- Young WF. (1997) Primary aldosteronism: Update on diagnosis and treatment. The Endocrinologist 7, 213–221. [Google Scholar]