Abstract
Purpose of Review
Biomarkers are commonly used in epidemiological studies to assess metals and metalloid exposure and estimate internal dose, as they integrate multiple sources and routes of exposure. Researchers are increasingly using multimetal panels and innovative statistical methods to understand how exposure to real-world metal mixtures affects human health. Metals have both common and unique sources and routes of exposure, as well as biotransformation and elimination pathways. The development of multi-element analytical technology allows researchers to examine a broad spectrum of metals in their studies; however, their interpretation is complex as they can reflect different windows of exposure and several biomarkers have critical limitations. This review elaborates on more than 500 scientific publications to discuss major sources of exposure, biotransformation and elimination, and biomarkers of exposure and internal dose for 12 metals/metalloids, including 8 non-essential elements (arsenic, barium, cadmium, lead, mercury, nickel, tin, uranium) and 4 essential elements (manganese, molybdenum, selenium, and zinc) commonly used in multi-element analyses.
Recent Findings
We conclude that not all metal biomarkers are adequate measures of exposure and that understanding the metabolic biotransformation and elimination of metals is key to metal biomarker interpretation. For example, whole blood is a good biomarker of exposure to arsenic, cadmium, lead, mercury, and tin, but it is not a good indicator for barium, nickel, and uranium. For some essential metals, the interpretation of whole blood biomarkers is unclear. Urine is the most commonly used biomarker of exposure across metals but it should not be used to assess lead exposure. Essential metals such as zinc and manganese are tightly regulated by homeostatic processes; thus, elevated levels in urine may reflect body loss and metabolic processes rather than excess exposure. Total urinary arsenic may reflect exposure to both organic and inorganic arsenic, thus, arsenic speciation and adjustment for arsebonetaine are needed in populations with dietary seafood consumption. Hair and nails primarily reflect exposure to organic mercury, except in populations exposed to high levels of inorganic mercury such as in occupational and environmental settings. When selecting biomarkers, it is also critical to consider the exposure window of interest. Most populations are chronically exposed to metals in the low-to-moderate range, yet many biomarkers reflect recent exposures. Toenails are emerging biomarkers in this regard. They are reliable biomarkers of long-term exposure for arsenic, mercury, manganese, and selenium. However, more research is needed to understand the role of nails as a biomarker of exposure to other metals. Similarly, teeth are increasingly used to assess lifelong exposures to several essential and non-essential metals such as lead, including during the prenatal window.
Summary
As metals epidemiology moves towards embracing a multi-metal/mixtures approach and expanding metal panels to include less commonly studied metals, it is important for researchers to have a strong knowledge base about the metal biomarkers included in their research. This review aims to aid metals researchers in their analysis planning, facilitate sound analytical decision-making, as well as appropriate understanding and interpretation of results.
Keywords: Metals, Review, Biomarkers, Arsenic, Barium, Cadmium, Lead, Mercury, Nickel, Tin, Uranium, Manganese, Molybdenum, Selenium, Zinc
Introduction
Epidemiological studies traditionally examine exposure-outcome relationships one at a time. With the advancement of novel analytical and statistical methods and the development of programs such as the Human Health Exposure Analysis Resource (HHEAR) to support the measure of multiple exposures [1], the field of environmental epidemiology is quickly moving to study environmental exposures in ways that more closely resemble real-life exposure mixtures [2–4].
Exposure to metals and metalloids (referred to throughout as “metals” for simplicity) is widespread, and four of the top ten chemicals of major public health concern recognized by the World Health Organization are metals (arsenic, cadmium, lead, and mercury) [5]. Chronic exposure to certain metals contributes to the development of adverse health outcomes across the life course, including but not limited to cardiovascular disease [6], kidney disease [7–9], some cancers [10, 11], adverse birth outcomes [12–14], neurocognitive outcomes [15], and bone disease endpoints [16].
Biomarkers are commonly used in epidemiological studies to assess metals exposure and estimate internal dose, as they integrate multiple exposure sources, including air, water, and food. Multi-element techniques such as inductively coupled plasma mass spectrometry (ICP-MS) can quantify metal levels with high sensitivity and specificity. In addition, relatively new tools such as Bayesian Kernel Machine Regression [17] and Weighted Quantile Sum Regression [18] enable the statistical analysis of large panels of metals. The interpretation of those analyses, however, can be complex. Metals have both shared and unique sources and routes of exposure (Fig. 1); some metals share common absorption, transformation, or elimination pathways, while others differ in their metabolic route; and not all biomarkers are effective measures of metals exposure. As metals epidemiology embraces a multi-metal/mixtures approach and metal panels expand to include less commonly researched elements, such as nickel, tin, and molybdenum, it is important for researchers to have a strong knowledge base about the metals studied.
Figure 1. Graphic summary of the main sources of exposure for the metals included in the review.
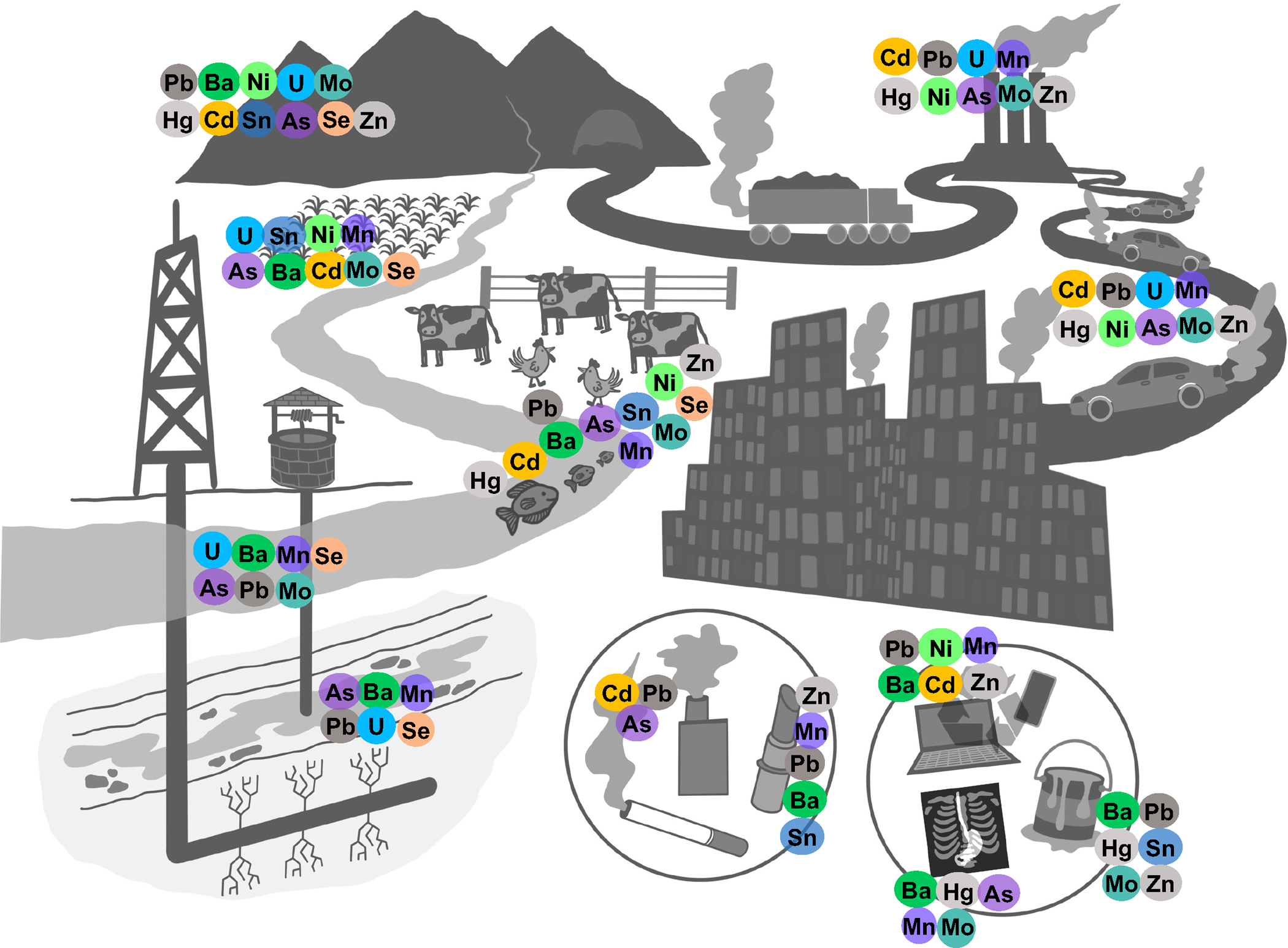
In this review, we discuss sources of exposure, biotransformation and elimination, and biomarkers of exposure for 12 metals, including 8 non-essential elements (arsenic (As), barium (Ba), cadmium (Cd), lead (Pb), mercury (Hg), nickel (Ni), tin (Sb), uranium (U)) and 4 essential elements (manganese (Mn), molybdenum (Mo), selenium (Se), and zinc (Zn)), commonly used in multi-metal epidemiologic analyses. This review aims to aid metals researchers in their analysis planning, analytical and statistical approaches, and interpretation of results.
Methods
Search Strategy and Data Abstraction
To identify the most informative literature for the metals included in the review, we searched across PubMed, Google-Scholar, and EMBASE using free text, key words, and MeSH terms. A separate search was conducted for each section (i.e., sources of exposure, biotransformation, and biomarkers), and refined for every metal, consistently including MeSH terms such as “biomarkers”[MeSH Terms], “biotransformationmation” [MeSH Terms], “absorption” [MeSH Terms], and “metabolism” [MeSH Terms]. No language or time window restriction was applied. We excluded non-original research, case reports, case series, and non-peer-reviewed literature except from institutional reports (i.e., Center for Disease Control and World Health Organization reports). We included a comprehensive selection of systematic reviews, meta-analyses, randomized controlled trials, and observational studies. If available, we primarily relied on longer follow-up, larger sample size, and more recent human studies. In vitro and animal studies were included to elaborate on emerging biomarkers. After the inclusion and exclusion criteria, a total of 521 unique records were identified, including laboratory, animal, and epidemiological studies.
Results
Non-essential Metals
Table 1 includes a summary of the most commonly used biomarkers, the exposure period reflected, and other considerations for the non-essential metals included in this review. Metals are presented alphabetically. Figure 1 displays their major sources of exposure.
Table 1. Summary of the most commonly used biomarkers for non-essential metals, their timing and important considerations when including in a study.
Timing is divided into 4 categories according to the half-lives of the biomarkers in the body: Acute (1–3 days prior), Recent (up to 1 month prior), Sub-Chronic (1 to 6 months prior), Chronic (>6 months prior).
| Metal | Biomarker | Timingc | Established Biomarker | Considerations |
|---|---|---|---|---|
| Arsenic | Whole Blood | Recent | Yes | Requires speciation of metabolites to aid assessment of toxicity Has analytic limitations |
| Urinea | Recent | Yes | Requires speciation of metabolites to aid assessment of toxicity | |
| Hairb | Sub-Chronic | Validated protocols for sample processing are needed. High interindividual variability in growth rate Predominantly reflect inorganic As |
||
| Toenail/Fingernailb | Sub-Chronic/ Chronic | Yes | ||
| Placenta | Sub-Chronic | Unclear | Limited studies | |
| Barium | Urinea | Acute | Yes | Limited studies |
| Feces | Recent | Unclear | Limited studies | |
| Cadmium | Whole Blood | Recent and Sub-Chronic | Yes | Reflects ongoing and short-term exposure but has a long-term component too |
| Red Blood Cells | Recent and Sub-Chronic | Yes | Reflects exposure over past 100 days. Similar half-life as whole blood |
|
| Urinea | Chronic | Yes | Reflects long-term exposure Low intraindividual variability Renal injury can limit validity |
|
| Hairb | Unclear | No | Window of exposure relfected is unclear | |
| Toenail/Fingernailb | Unclear | No | Window of exposure relfected is unclear | |
| Placenta | Sub-Chronic | Unclear | Correlates well with maternal blood and urinary Cd | |
| Feces | Recent | Unclear | Captures dietary intake | |
| Liver/Kidney | Chronic | Unclear | Low sensitivity Reserved for occupational biomonitoring |
|
| Lead | Whole Blood | Recent | Yes | Good indicator in low exposed populations |
| Cumulative Blood Lead Index | Chronic | Yes | Integrated measurement of average blood Pb levels over time | |
| Urine | Unclear | No | Not a good biomarker | |
| Hairb | Unclear | No | Window of exposure relfected is unclear | |
| Toenail/Fingernailb | Unclear | No | Window of exposure relfected is unclear | |
| Placenta | Sub-Chronic | Unclear | Correlates well with maternal blood concentrations, inconsistent findings for cord blood | |
| Bone | Chronic | Yes | Non-invasive Intra-individual and inter-laboratory variability |
|
| Teeth | Chronic | Yes | Decidious and adult teeth reflect different windows of exposure. | |
| Chelatable urine | Chronic | Requires intravenous EDTA | ||
| Mercury | Whole Blood | Recent | Yes | Indicator of total mercury, but mostly organic mercury in populations with seafood intake Valid as measurement of internal dose in chronically exposed populations Indicator of pre-natal exposure in maternal blood |
| Red Blood Cells | Sub-Chronic | Yes | Indicator of organic mercury | |
| Urine | Recent | Yes | Indicator of inorganic mercury Represents internal dose in chronically exposed populations Can also reflect demethylated organic mercury |
|
| Hairb | Chronic | Yes | Indicator of organic mercury Indicator of pre-natal exposure (maternal hair) |
|
| Toenail/Fingernailb | Sub-Chronic | Yes | Indicator of organic mercury | |
| Placenta | Sub-Chronic | Unclear | Emerging biomarker | |
| Nickel | Plasma/Serum | Acute | Yes | Reflect exposure to soluble Ni compounds |
| Urine | Acute | Yes | High correlation with serum Ni | |
| Tin | Whole Blood | Recent | Yes | Speciation needed for organic forms |
| Urine | Chronic | Yes | Indicator of inorganic tin, after digestion with acid and oxidation, extracting (Sn+4) | |
| Bone | Chronic | Unclear | Only invasive techniques available | |
| Uranium | Urine | Acute and Recent | Yes | Uranium isotopes can be determined Can reflect chronic exposure if exposure is constant |
| Hairb | Sub-Chronic | Unclear | High correlation with nail U | |
| Toenail/Fingernailb | Sub-Chronic | Unclear | High correlation with hair U | |
| Bone and soft tissue | Chronic | Unclear | Only invasive techniques available |
Needs adjustment for urine dilution (creatinine or specific gravity)
Sample requires environmental decontamination
Timing refers to the exposure window that the biomarker reflects relative to sample collection.
Arsenic
Arsenic is a metalloid found in inorganic (arsenite and arsenate) and organic (arsenobetaine, arsenosugars, monomethyl, and dimethyl As) forms. Their toxicity, metabolism, sources of exposure, and effects on human health greatly differ [19, 20]. Because inorganic arsenicals and their methylated metabolites are considerably more toxic than the organics (especially seafood arsenicals), we address them separately.
Major Sources of Exposure
Inorganic As can be released into water from the natural erosion of rocks and soil [19, 21] and can be taken up by plants’ root systems [22]. Inorganic As contamination of soil and water is also a result of anthropogenic activities, such as mining; coal-burning power plants; smelting operations [23]; wood preservatives; agriculture, where it was used as a pesticide prior to 1960 [24]; urban runoff, and various industrial waste sources [19]. These activities can lead to the mobilization of As from soil, landfills, or residue deposits into ground and surface water [19, 25–30]. Inorganic As can also be found in air as a mixture of arsenite and arsenate suspended as particulate matter [31], although non-occupational exposure via air is thought to be minimal in most populations, except for areas with As-contaminated coal burning [19]. The major route of exposure to inorganic As is through the ingestion of contaminated drinking water and food. More than 100 million people worldwide rely on water contaminated with inorganic As above 10 μg/L [32] (the WHO, US Environmental Protection Agency, and European Union standard), including 2 million people in the US [20], above 10 μg/L (the WHO, US Environmental Protection Agency, and European Union standard). When water As exposure is low, food is the major source of exposure [33, 34]. The primary contributors to dietary inorganic As intake are vegetables, fruits and fruit juices, and rice [35, 36]. Organic As is found in fish and shellfish, predominantly in the forms of arsenobetaine, arsenosugars, and arsenolipids [37]. It is also a component of some herbicides and anti-microbial additives such as roxarsone and nitarsone, which were used to increase efficiency in animal poultry feed [38]. The approval of As-based drugs in poultry production was withdrawn by the FDA in 2016 in the USA, after it was found that these arsenicals could transform into inorganic As [39]. Additionally, organic As is the active principle of antiparasitic drugs such as melarsoprol, which is used as a treatment for trypanosomiasis (sleeping sickness) and leishmaniasis [40]. Melarsoprol and inorganic arsenic are also used in the treatment of certain types of leukemia, myeloma, and other neoplasia [41, 42]. Organic As in air is rare, except in areas with intensive use of arsenical pesticides [43].
Absorption, Biotransformation, and Elimination
Inorganic arsenic is absorbed in the gastrointestinal (GI) tract, transformed through a series of methylation and reduction processes, and excreted primarily through the urine [19]. Throughout the metabolic process, species with different toxicities and elimination rates are formed. Trivalent and pentavalent inorganic arsenicals undergo two sequential methylation reactions to form monomethyl (MMA) and dimethyl (DMA) As. Inorganic and monomethylated forms of As have higher cytotoxic activity [44]. Methylation occurs mostly in the liver and requires the enzyme As methyl transferase (AS3MT), with S-adenosyl methionine as the methyl donor. Once formed, DMA and to a lesser extent MMA are rapidly excreted in urine. Methylation plays a crucial role in increased As elimination and decreased tissue retention [20, 45]. Organic As species such as arsenobetaine and arsenocholine are not metabolized, do not enter cells, and are excreted mostly unchanged in the urine [46]. Arsenosugars on the other hand have a complex metabolism and can be transformed into up to 12 As metabolites, including DMA, oxo-DMA, and rare species such as oxo-DMAA, thio-DMAA, thio-DMAE, and thio-arsenosugar [47, 48]. Their toxicity is believed to be limited [49]. Urinary excretion is the main route of elimination for both inorganic and organic As species. Inorganic As is excreted in three phases: the majority is eliminated within the 4 first days after exposure, and 2 longer-term components eliminated at 1 week and up to 38 days [50].
Genetic variants in AS3MT have been associated with different As elimination rates and variable profiles of inorganic and methylated As species in urine, suggesting a genetic determination of As metabolism capacity [19]. Arsenic methylation is strongly influenced by the availability of methyl groups and co-factors involved in the one-carbon metabolism pathway, which are provided by nutrients, including vitamins B2, B6, B9 (folate) B12, choline, and betaine [51]. Nutritional deficiencies of B vitamins have been associated with increased toxicity of inorganic As in humans [52]. Supplementation with folic acid, other methyl donors, and compounds that interact with one-carbon metabolism have been associated with an increased metabolism and excretion rate of inorganic As in randomized clinical trials [53, 54]. Sex differences in As metabolism are well known. Women have a greater As methylation capacity compared with men, likely due to their higher phosphatidylethanolamine N-methyltransferase activity, which is involved in one-carbon metabolism and regulated by estrogen [55, 56]. For the same reason, As metabolism rapidly increases during pregnancy [57, 58]. Children have been reported to have a more efficient methylation activity than their parents [59, 60], potentially because of a more efficient second methylation step in children compared with adults [61].
Biomarkers
Total As in urine is one of the most commonly used biomarkers of As exposure in epidemiologic studies [62]. Several studies have reported strong correlations between urinary As and As in drinking water and rice intake [63–65]. Given the elimination phases of As, urinary As primarily accounts for exposures that occurred within the previous month [66, 67].
Importantly, total urine As reflects exposure to both organic and inorganic As. Thus, in populations with fish and seafood intake, total urinary As may primarily reflect organic arsenicals, in which case this biomarker will not reflect toxic inorganic As species well [68]. Therefore, when fish and seafood intake is prevalent, As speciation and adjustment for arsebonetaine is needed to distinguish between inorganic and organic arsenicals [69–71]. In the absence of seafood intake, the general proportions of As metabolites in urine have been reported to range from 10 to 30% for inorganic As, from 10 to 20% for MMA, and from 60 to 70% for DMA [44]. Similar to urinary As, blood As also reflects both organic and inorganic As. However, As speciation in blood is more complex than that in urine because concentrations are generally much lower [19]. In the absence of seafood consumption, the proportions of As metabolites in blood are different from those found in urine. Previous studies have reported the following proportions for blood: 43% for DMA, 30% for MMA, 13% for As III, and 13% for As V [72]. Inorganic arsenic travels through the blood and has a high affinity for keratin-rich tissues [50]. Hair, clipping from all ten toenails, and/or fingernails are used as biomarkers of past exposure. Fingernails generally represent exposures that occurred in the previous 6 months, while toenails, which have a slower growth rate, represent exposures from the previous 6 to 12 months [20]. For hair, every 1 cm from the scalp represents exposure from a prior month [73]. Environmental decontamination is needed to obtain reliable measurements from these tissues [19]. Moderate to high correlations have been reported between toenail, fingernail, hair, and urine As concentrations, particularly in populations with high levels of inorganic As exposure [74]. Increasing evidence suggests that a toenail sample at a single point in time could represent a good biomarker for long-term exposure, when external exposures are consistent. However, validated protocols are needed to reduce measurement error from analytical methods in As toenail determination [19, 74]. Inter-individual differences in toenail growth rates may also contribute to differences in the time window of exposure that is reflected by the biomarker. Although few studies have investigated placental As as a biomarker of exposure, a study of private well users in the USA reported positive and significant correlations between As concentrations in the placenta and As concentrations in maternal urine, maternal and infant toenails, and drinking water [75].
Barium
Barium is rarely found in its inorganic form. It is usually combined with other elements, forming species such as barite (BaSO4), Ba chloride (BaCl2), or carbonates (e.g., Ca (Ba) CO3). These species have different solubilities, which has implications for their bioavailability [76, 78].
Major Sources of Exposure
Barium is an alkaline metal that occurs naturally in the earth crust, rocks, and coal [76, 78, 79]. It can be released into water from natural erosion. Traces of Ba from natural sources are ubiquitously present in food and water. Recent anthropogenic activity has intensified the burden of exposure to this metal [80]. Several activities, particularly petroleum and steel industries, drilling activity, and shale gas exploration [78], have increased the amount of Ba in soil, water, and air. Barium compounds are also used in the production of plastics, electronic devices, bricks, paper, glass, and cosmetics [77, 81, 82], pigments, aluminum, and sugar refining and fertilizers [76, 83]. In the last 40 years, Ba has also been widely used as a contrast medium in esophageal and GI tract function testing, and as a treatment for lower GI tract bleeding, among other medical applications [84, 85]. The main route of Ba exposure in the general population is through ingestion of contaminated food and water [76, 78], followed by inhalation from ambient air [76, 78]. Plants that grow in soil with high Ba concentrations can accumulate this metal, contributing to dietary intake [86]. Major dietary sources of Ba exposure include legumes and nuts, such as pecans, peanuts, and brazil nuts, which may contain Ba concentrations as high as 4,000 μg/g [76, 77, 87–90]. Other foods such as cabbage, soybeans, dairy products, and some processed foods have high Ba concentrations [77, 88, 91, 92]. Ba levels in water are variable. Ba in groundwater is frequently associated with radium, adding its toxicity to radium radionuclides such as 226Ra and 228Ra, with half-lives of more than 1000 years and 6 years respectively [78]. Ba is commonly found in urban air pollution particulates (mostly as BaCl2 and BaSO4) due to fuel exhaust and waste burning[76].
Absorption, Biotransformation, and Elimination
The absorption of Ba in the GI and respiratory tracts depends on the solubility of its chemical form. For example, highly soluble species such as BaCl2 and BaCO3 are more likely to be absorbed than others (e.g., BaSO4). The absorption of Ba compounds into the bloodstream is highly variable and ranges from 1 to 60% of total intake [76], with higher absorption rates observed after longer durations of exposure [93] and among fasting individuals. In contrast, Ba absorption decreases if there is a high concentration of competitive ions in the GI tract, such as calcium, phosphorus, and zinc [78]. Substances such as sodium alginate [94] have also been shown to reduce Ba absorption in the GI tract; in contrast, lysine and lactose [95] facilitate its absorption. Ba prevents calcium absorbance in pancreatic islets, which can lead to an increased release of insulin [96]. Ba does not undergo metabolic transformation in the body [62]. Once absorbed, it is rapidly cleared from the bloodstream, with 90% distributed to bone, followed by teeth, heart, lung, skin, adipose, connective, and other tissues [76, 97]. Barium has relatively short half-life and is eliminated from the body within 3 to 42 days; the half-life of barium in bone tissue is 50 days. Ninety percent of Ba administered intravenously for medical procedures as well as for other routes of absorption is excreted into feces and urine within this period [76, 97], with ~ 75% eliminated within 3 days. In the kidney, Ba is reabsorbed in the renal tubules, which decreases its urine excretion rate [77]. The rate of fecal Ba elimination is at least 2–3 times higher than the elimination through urine, and it is mediated by mechanisms such as pancreatic secretions, saliva, or direct excretion through the intestine wall; biliary excretion is only responsible for a small fraction of its fecal elimination [98]. Ba can act as an antagonist of potassium, and vice versa, by blocking the potassium channels of sodium–potassium pumps located in cell membranes [99], which reduces the concentration of extracellular potassium leading to hypokalemia [92]. Potassium can be used as an antidote for acute Ba intoxication [100–102].
Biomarkers
Barium can be detected in blood, urine, feces, and other biological tissues such as the placenta, teeth, and bone [77, 103–107]. Fecal and urinary Ba concentrations reflect recent exposure, within the previous 3 days and up to 2 weeks [97]. Overall, there is a need for studies which investigate correlations between environmental Ba concentrations and levels in urine and other biomarkers [76, 78, 108].
Cadmium
Major Sources of Exposure
Cadmium can naturally occur combined with other metals such as Zn, Pb, and copper in Cd-containing ores [109]. Human activities, including mining, smelting, industrial production of batteries, plastic and solar cells [110], fossil fuel and waste burning, and phosphate fertilizers, are the main sources of environmental Cd and the primary sources of soil and water Cd-contamination [90, 111]. Plants, including tobacco, can bioaccumulate Cd when they are grown in contaminated soils, incorporating this toxicant into the food chain and tobacco products [112, 113]. Tobacco smoke is a major source of Cd exposure. It is estimated that exposure to Cd in smokers is at least 10 times higher than in nonsmokers, adjusting for dietary intake [111]. Cd exposure can also occur as a result of secondhand smoke [114]. Decreases in population mean blood Cd levels have been reported in epidemiologic studies following reductions in smoking prevalence [20, 115]. The main source of Cd exposure among non-cigarette smokers is diet. Green-leaf vegetables such as spinach and lettuce and grains and seeds such as peanuts, soybeans, and sunflower seeds contain particularly high concentrations of Cd [111]. Consumption of shellfish and offal/ organ meat also contributes to Cd exposure [116, 117]. Cadmium can also be found in air as a component of particulate matter air pollution, especially in areas with high levels of ambient air pollution, such as urban areas and areas with heavy industrialization [118].
Absorption, Biotransformation, and Elimination
Cadmium absorption rates through the GI tract and inhalation are variable and range from 1 to 25% [111, 119, 120]. Absorption rates are influenced by several factors, including particle size for inhalation exposures, the solubility of certain Cd salts in biological fluids (e.g., Cd chloride is highly soluble), and the availability of cellular transporters. In drinking water, Cd is typically found in its ionic form, while in food it is usually linked to proteins (e.g., metallothionein) and other molecules [111]. Cadmium can use several transporters, including zinc and iron transporters, calcium channels, glutathione transporters, and divalent metal transporters (DMT1), which facilitate Cd absorption [111]. An inverse correlation between body iron and Cd absorption has been identified by previous studies [121]. While the molecular mechanisms are unclear, a dysregulation of DMT1, a metal transporter protein in the GI tract may partially explain this phenomenon. On average, the accumulation of Cd is higher in women compared with men at equal exposure levels, possibly due to differences in DMT1 expression and to the lower iron stores in women compared to men [20, 121, 122]. Zinc supplementation can reduce Cd absorption in the GI tract and induce the synthesis of metallothionein and a favorable redox homeostasis, suggesting a potential antagonist effect on Cd [123]. A recent study identified a 28% heritability for urinary Cd concentrations. Genetic variants linked to an increased expression of glutathione transporter ABCC1 may have a role in individual susceptibility to Cd absorption and internal dose [124]. Cadmium does not undergo metabolic transformation. Once it enters the bloodstream it primarily binds to metallothionein, forming metallothionein-Cd complexes. These complexes are distributed through the body, accumulating in the liver, bones, and kidneys [125].
In the kidney, [90, 111, 126] metallothionein is filtered by the glomeruli and reabsorbed in the proximal renal tubule, where free ionic Cd is released into the kidney cortex. Ionic Cd can cause renal toxicity by inducing oxidative stress, inactivation of metal-dependent enzymes, and activation of calmodulin and other enzymes, among other mechanisms [111, 127]. When free Cd concentrations in the renal cortex exceed 50 μg/g, Cd cannot be captured by metallothionein, causing nephrotoxicity. Less than 0.01% of the Cd body burden is eliminated by urinary excretion every day, which leads to a chronic accumulation of Cd in the kidney. This accumulation usually peaks after 50 to 60 years of exposure and then stabilizes due to the onset of Cd-induced renal injury, as well as the physiological loss in the renal cortex due to aging, which results in higher excretion of Cd in urine [128]. The half-life of Cd in the kidney is estimated to range between 6 and 38 years [129]. In contrast with chronic exposure, short-term exposure to Cd does not typically cause visible impacts on renal function. Cadmium also accumulates in the liver, where its half-life is estimated to range from 4 to 19 years [111].
Biomarkers
Cadmium can be measured in blood, urine, feces, placenta, and preferentially in soft tissues, including the liver and kidneys. Blood Cd is commonly used to assess recent exposure. Cd accumulates in erythrocytes and its half-life is around 100 days [20]. However, there is a slow component in blood, for which the half-life is 7–16 years; this component can also be indicative of internal body burden [90, 111]. Urine Cd is a biomarker of total body burden and long-term cumulative exposure due to its accumulation in the renal cortex for years and its slow rate of elimination in urine [130, 131]. However, in populations where kidney function is compromised (e.g., renal injury from Cd-induced damage, age, other comorbidities) urinary Cd should be interpreted with caution. In these populations, the rate of Cd excretion increases, and urinary Cd no longer reflects body burden [111, 132]. Previous studies have documented a high correlation between total Cd exposure and urinary Cd concentrations [133–135] and low intra-individual variability, even in populations exposed to low levels [132, 136, 137]. Therefore, a single spot urine measurement can likely be used as a reliable biomarker of chronic exposure. Emerging biomarkers to detect early stages of Cd-induced nephrotoxicity include urinary measures of Kim-1 and beta2-microglobulin. However, their use is still generally limited to in vitro and animal studies [127]. Fecal Cd is reported to be a good biomarker of dietary Cd intake and indicates recent exposure, reflecting a combination of absorbed Cd, which is eliminated at a similar rate in urine and feces, and unabsorbed Cd from the diet [111]. Hair, fingernail and toenail Cd concentrations are not good biomarkers of exposure and are poorly correlated with smoking. The use of nails as biomarkers of exposure has been implemented in pilot studies identifying a low correlation between nail and blood Cd [138]. Cadmium levels in organs including the liver and kidneys can also be directly assessed using non-invasive techniques such as X-ray fluorescence analysis or neutron activation analysis. However, these measurements are limited by low sensitivity and are usually reserved for monitoring highly exposed workers rather than the general population [111]. Maternal smoking and Cd concentrations in both maternal blood and urine have each been associated with placental Cd concentrations across multiple studies [139–147]. In contrast, correlations between placental and cord blood Cd have been less consistent, likely because the placenta serves as a partial barrier to this metal; placental Cd concentrations have been reported to be higher than in maternal blood [139, 143, 148].
Lead
Major Sources of Exposure
The general population is exposed to Pb from air, drinking water, food, and indoor dust [149]. Lead does not undergo degradation over time in the environment. As a result, most individuals, particularly those living in urban areas, are exposed to high levels of Pb from previous decades’ emissions. Before the 1990s, the main sources of Pb exposure were the combustion of leaded gasoline and Pb-based paint. Although Pb fuel additives were banned in 1995 in the USA [150], some are still in use for farm equipment, aircraft, and marine engines [149, 151, 152]. Other sources of exposure include waste, coal and oil burning, renovation of historical Pb-based paints or Pb-based paints in older housing, tobacco products, secondhand smoke, cosmetics, and electronic products [20]. The increasing amount of e-waste (discarded electronic devices) is also an emerging source of metal exposure, from dumpsites and informal recycling activities for Pb, Hg, and other toxicants [153]. Lead contamination in drinking water is mainly due to the corrosion of Pb-based pipes [154, 155]. Lead has also been documented in certain foods and herbs, including spices imported into the USA, as a result of athmospheric deposition, bioaccumulation from contaminated soils, and adulteration during processing and preparation [149, 156, 157]. While Pb concentrations in plant-based foods in the USA are usually low, this route of exposure is particularly relevant for children under 5 years, as Pb contamination has been found in some formulas, infant toddler cereal, cocoa powder and chocolate products, and candy ingredients and wrappers [20, 158–160]. Preparation of baby formula with contaminated tap water also contributes to this burden of exposure [149]. Soil and dust ingestion of Pb is particularly relevant for young children because of the high ingestion rate related to repetitive mouthing behavior and hand contamination [161–166]. Consumption of fish and shellfish captured in water with high Pb contamination is also a relevant source [149]. The burden of exposure is higher for populations living in urban areas and those close to sites with Pb emissions (e.g., smelters, Pb battery recycling plants, and mines) and accumulation from other industrial activities. In the USA, the reference value for blood Pb in children was updated to 3.5 ug/dL in 2021 [167]. However, no safe level of blood Pb for human health has been identified [168].
Absorption, Biotransformation, and Elimination
The absorption rate from inhalation of inorganic Pb is almost 100% for small particles (< 2.5 μm) [149]. The absorption rate in the GI tract is variable. Adults absorb between 3 to 10% of ingested Pb, while children can absorb up to 50% [169–172]. Fasting status [146, 169–171, 173–175] and iron deficiency [176, 177] contribute to higher Pb absorption [178, 179]. Dermal absorption has been documented in animal models, but its contribution to body burden is minimal [149]. In the bloodstream, 99% of Pb enters red blood cells where it binds to various proteins [149]. Sigma-aminolaevulinic acid dehydratase (ALAD) has the highest affinity for Pb; the binding of Pb displaces zinc from this protein, impairing its function [20, 180]. The majority of lead is transported from blood into bone tissue, which accumulates 90% of the body burden. In the bone, Pb can replace calcium and bind phosphate, forming stable complexes that substitute the original calcium-phosphate salts within the bone matrix [181]. The accumulation of Pb in the bone is age-dependent and occurs mostly in areas that are undergoing calcification and remodeling at the time of exposure [182]. As a result, Pb primarily accumulates
in the trabecular bone of children and in cortical and trabecular bone in adults, where it can remain for more than 30 years [183]. From the bone, Pb can be transported back into the bloodstream and into other tissues when bone is resorbed [20]. Physiological and pathological processes that increase bone resorption, including pregnancy, menopause, pronounced weight loss, and osteoporosis, increase Pb concentrations in blood [20, 149, 184–189]. Organic Pb is metabolized in the liver where it undergoes oxidative dealkylation by P-450 enzymes. The main elimination routes are urine and feces, although excretion is very low [149]. Lead can also be eliminated in smaller proportions through sweat, saliva, hair, nails, and seminal fluids [190–198]. Lead can cross the placenta and is excreted in human milk, resulting in potential sources of Pb exposure during vulnerable developmental windows [199, 200].
Biomarkers
Total Pb can be measured in blood, bone, teeth, nails, hair, and urine [201, 202]. Whole blood Pb is the most used biomarker for recent and long-term exposures. If exposures are constant, one spot measurement of blood lead can be a reasonable indicator of long-term exposure [20, 149]. Although only 2% of the body burden of Pb is stored in blood [149], there is a constant recirculation of Pb from bone into the bloodstream. Blood Pb therefore reflects an integrated mixture of exposure from the past few months and has a longer-term exposure component over the years [20, 203]. However, short-term changes in exposure can rapidly influence blood levels of Pb. To understand the burden of chronic Pb exposure, the cumulative blood Pb index (CBLI) can be calculated [149]. This index is based on repeated blood samples over time and provides an integrated measurement of average blood Pb levels over that time period [20, 201, 204–212]. Lead can be measured in bone tissue using noninvasive procedures such as K-Shell and L-Shell X-ray fluorescence (XRF) [213–215]. This is a biomarker of cumulative exposure since the primary reservoir of Pb in the body is bone. Accumulation of Pb in trabecular bone is preferably measured in the patella, calcaneus, and sternum; Pb in cortical bone is measured in the tibia, phalanx, or ulna [149]. Previous studies have reported high correlations between bone Pb measured by XRF and CBLI [20, 208]. However, this biomarker has important limitations, as several factors, including bone mass, body mass index, gender, age, and inter-laboratory variability may affect its accuracy [216]. Furthermore, even at low doses, XRF involves the use of radiation [182]. Chelatable Pb, which is measured using chelating agents such as dimercaptosuccinic acid (DMSA) and ethylenedi-aminetetraacetic acid (EDTA) administered prior to urine collection, is considered a biomarker of bioavailable Pb stores and Pb body burden. Chelation with EDTA requires intravenous administration [217, 218]. The best time for measuring chelatable Pb is 3–4 h after administering the chelating agent [219].
Several laboratory techniques have been developed over the past decades to allow the measurement of Pb and other metals in teeth dentine and enamel [220–223]. Teeth are a minimally invasive biomarker that can provide chronological information on cumulative exposure across the life course. Deciduous teeth can be used to assess exposures during the prenatal period and childhood [224, 125, 226]. Previous studies in children have reported strong correlations between dentine Pb and blood Pb concentrations (Spearman’s p = 0.64, p < 0.0001) and cord blood Pb (Spearman’s p = 0.69, p < 0.0001) [225, 227] [149] Placental Pb correlates well with maternal blood Pb [30, 228] and with Pb concentrations in drinking water [228]. However, similar to Cd, correlations between placental Pb and concentrations in cord blood have been inconsistent [229]. Hair, nails, urine, and plasma are not established biomarkers for measuring the internal dose of Pb; previous studies have reported low correlations between environmental Pb exposure, bone Pb, and Pb concentrations in these biospecimens, particularly at lower concentrations, therefore the potential exposure windows reflected by these biomarkers remain unclear [20, 230]. Urine and plasma Pb may be acceptable among populations exposed occupationally or at higher levels [230]. However, their utility for general populations is unclear.
Mercury
Major Sources of Exposure
Mercury can be found naturally in the environment as a result of volcanic activity and corrosion of rocks containing Hg [231, 232]. However, environmental contamination and resulting exposure to Hg occurs mostly due to anthropogenic activities, such as mining and smelting, coal combustion in thermal power plants, waste burning, and bioaccumulation of Hg in the food chain [233–236].
Mercury is found in several chemical forms. Humans are predominantly exposed to organic Hg (e.g., methylmercury) [237, 238], which can be found in seafood, poultry, a variety of pesticides, insecticides, thimerosal-containing vaccines, and in some herbal supplements and alternative medicines [239]. Plants can also bioaccumulate organic Hg from soil [240, 241]. Inorganic Hg (Hg + 2) is used as an additive in some prescription drugs, such as laxatives or diuretics, and other products including teeth powdering and certain cosmetics [237]. Inorganic Hg can also be an intermediate product of metabolized organic Hg [242]. Metallic Hg (Hg0, also known as elemental Hg), which is the only metal found in a liquid state at room temperature, has been used in dental amalgams in the past, [243, 244], as well as in thermometers and as an additive to paint and other materials [237, 245]. Metallic Hg evaporates easily, contributing to human exposure through inhalation [235].
The main route of Hg exposure in the general population is oral ingestion from diet [246]. Fish consumption, which contains high levels of organic Hg, contributes to more than 90% of Hg exposure in the US [247]. Recent studies in populations that consume large quantities of rice have shown that Hg-contaminated rice may also contribute to exposure [248, 249]. Direct contact with metallic Hg used in medical equipment such as dental amalgams is a secondary route of exposure which occurs through inhalation of metallic Hg as a vapor [250]. Some cosmetic products like skin-lightening and whitening cosmetics may contain small amounts of inorganic Hg and constitute an additional source of exposure [251, 252]. The most relevant occupational exposures occur in metal and waste processing plants, manufacturers of electrical equipment and building materials, and medical professions, including dentists and other healthcare providers [236, 253, 254].
Absorption, Biotransformation, and Elimination
Organic Hg has the highest bioavailability of the three forms, being easily absorbed in the GI tract, with absorption via oral intake ranging between 50 and 100%. The absorption of metallic and inorganic Hg in the GI tract is minimal; however, more than 80% of inhaled metallic Hg is absorbed in the lungs entering the systemic blood circulation [255]. The organic and metallic forms of Hg can easily cross the blood–brain barrier, entering the central nervous system, which is a relevant target of Hg toxicity [256]. After absorption, both organic and inorganic Hg undergo a series of oxidation–reduction cycles [246]. Metallic Hg (Hg0) is mostly transformed into inorganic Hg in the bloodstream, where it enters red blood cells and it is oxidized to inorganic Hg (Hg + 2) by catalase enzymes [257]. This process also occurs in the lungs, the brain, and the liver [246, 258], and can be inhibited by substances such as ethanol, which can impair Hg uptake by red blood cells [259]. The metallic Hg that is not transformed into inorganic Hg is mostly eliminated through exhaled air in the lungs [246]. The main routes of elimination for organic Hg are the feces, followed by urine and hair; biliary elimination plays a particularly important role in the elimination of organic Hg and urinary excretion accounts for less than one-third of organic Hg elimination from the body. The half-life of organic Hg in blood ranges from 50 to 100 days. Hair mercury can reflect the levels of organic mercury in blood during the previous 100 days, with every cm of hair reflecting exposure during the previous month [246, 260, 261]. Inorganic Hg has a terminal half-life of approximately 1–3 months in the body [262]. The majority of organic and inorganic Hg forms accumulate in the kidneys and the brain, which are the main targets of Hg toxicity. The primary route of elimination for inorganic Hg is urine. The Hg urine elimination rate is representative of the overall body elimination rate of inorganic Hg [246]. Acute exposure to inorganic Hg has been shown to be followed by two elimination phases: an initial phase of rapid elimination of high Hg concentrations from tissues (4–5 days) [246, 263] followed by a slower phase that can be prolonged due to persistent accumulation of Hg in tissues such as the brain which can last for years [264]. Both inorganic and organic Hg can be excreted in human milk [265, 266]. Mercury levels in cord blood are higher than in maternal blood, indicating placental transfer of Hg [267, 268].
Biomarkers
Mercury can be measured in several biospecimens, with urine, hair, and blood being used most frequently [246]. It can also be measured in feces, human milk, nails, and placenta [269, 270]. The different chemical forms of Hg have implications for its distribution, toxicity, accumulation, and elimination. As a result, different biospecimens reflect exposure to certain forms of Hg more accurately than others [250]. Hair is the biomarker of choice for measuring chronic exposure to organic Hg, as keratin binds to organic Hg [271, 272] Fingernails and toenails similarly reflect organic Hg for the same reason. Clippings from all ten toenails reflect exposure over the previous 6 to 12 months [273]. Prior studies have shown a high correlation between hair concentrations of Hg and brain tissue and whole blood at a ratio of 250:5:1. This ratio is recommended by the WHO for converting hair Hg concentrations to blood Hg concentrations [274]. Similarly, high correlations between nail and hair Hg (r > 0.9, p < 0.05) [273] and dietary Hg (Spearman’s p = 0.48, p < 0.01)[275] have been reported. Hair is not a good biomarker for measuring the burden of exposure to metallic Hg (Hg0). To understand the distribution of Hg species (organic vs. metallic) in hair samples, measuring Hg-stable isotopic signature has been successfully used in previous studies [276].
During pregnancy, organic Hg concentrations in hair are highly correlated with fetal blood levels, indicating that it is a good biomarker for prenatal exposure [269]. Maternal nails collected in early pregnancy and at delivery are useful biomarkers of prenatal exposure to organic Hg over the previous ~ 3 months. Prior studies identified a high correlation between maternal nail Hg at parturition and hair segments at 0–3 cm from the scalp [273]. The utility of placental Hg as a biomarker may depend on the specific Hg species evaluated. A study in Sweden which measured inorganic Hg in the placenta and maternal blood and collected information on the number of dental amalgams reported moderate correlations with inorganic Hg in cord blood [277]. The same study also reported positive correlations between placental methylHg and concentrations in both maternal and cord blood and freshwater fish consumption. However, results have been less consistent for total Hg [30, 145, 148].
Urine Hg is considered the biomarker of choice for measuring exposure to inorganic Hg, both for the general population and for capturing occupational exposures [246]. Urine excretion reflects the internal dose of inorganic Hg accumulation in the kidney after acute exposure [274, 278], but also can be used as a measure of internal dose in chronically exposed populations [250]. Urine Hg can reflect exposure to inorganic Hg as well as to organic Hg that is de-methylated and excreted through the kidney. However, the proportion of organic Hg that gets demethylated and excreted in the urine remains unclear as previous studies have reported mixed findings [246, 279–282]. Blood reflects exposure to all chemical forms of Hg [250, 272, 283]. Organic Hg primarily accumulates in red blood cells where it binds to hemoglobin, whereas inorganic Hg is distributed between both red blood cells and plasma [283]. The half-life of Hg in blood is approximately 50 days [284]. As a result, blood Hg is a reliable biomarker of recent exposure, although it can also be used as a biomarker of long-term exposure in populations chronically exposed to this metal. Maternal blood Hg concentrations during pregnancy can be used as a biomarker of prenatal exposure [269].
Nickel
Major Sources of Exposure
Nickel is found in soil, dust, and the atmosphere as a result of natural emissions. Anthropogenic sources of Ni include waste burning, combustion of fossil fuels, and steel production, among others, which also results in soil, water, and air contamination [285]. Nickel is also used as an alloy compound in jewelry, coins, and stainless steel materials and in other industrial activities such as fabrication of electric appliances and batteries [286].
Diet is the main route of exposure to Ni, as Ni can bioaccumulate in plant species that grow in contaminated soils [287], followed by inhalation and dermal contact [288]. Certain vegetables, such as spinach, asparagus, carrots, broccoli and green beans, tomato, cocoa, chocolate, and nuts can contain high concentrations of Ni [287, 289]. Smoking also constitutes a source of exposure [287, 290]. Populations that live near refineries and industrial sources of Ni are at higher risk of exposure [291]. Similar to other metals, household dust contamination with Ni is of particular concern for children [292]. In recent years, there is also concern about the use of metallic coils which contain Ni (nichrome) as an additional exposure among users of e-cigarette devices [293, 294].
Absorption, Biotransformation, and Elimination
The absorption rate of ingested Ni is highly variable and ranges from 2 to 30% [285, 286]. Approximately 20 to 35% of inhaled Ni is absorbed in the lungs. The solubility of Ni compounds has an impact on their absorption through cell membranes [288, 295]. While soluble Ni compounds tend to be easily absorbed by diffusion or transported by calcium channels or divalent cation transporters, insoluble particles can only be absorbed by phagocytosis [296, 297]. Fasting status and iron deficiency may also increase Ni absorption [286, 298] in the GI tract. Dermal absorption is generally low because little ionized Ni crosses the skin barrier [299]. This absorption increases with skin abrasions and can be relevant in occupational settings, particularly those where Ni nanoparticles are manipulated [300]. Dermal contact also leads to symptoms of contact dermatitis in 10–20% of the population [286]. In the bloodstream, Ni primarily binds to albumin, but also can be found in combination with amino acids and peptides [300]. The main elimination pathways for all sources of Ni exposure are urine and feces [285], and its half-life and accumulation in the body is relatively short, from 12 to 48 h [286, 301]. Nickel primarily accumulates in the lungs and the kidney, but can also accumulate in the heart, diaphragm, and spinal cord [302]. Magnesium and zinc can accelerate the elimination rate of Ni and decrease its systemic accumulation [303]. Cadmium and chromium can interact synergistically with Ni, increasing its toxicity [304, 305]. The use of chelation agents such as triethylenetetramine (TETA) and Cyclam, have been used to treat Ni toxicity successfully [286]. Nickel is also chelated by EDTA [306].
Biomarkers
As soluble Ni compounds are more likely to be absorbed, serum and plasma Ni reflect exposure to soluble Ni compounds most accurately [286]. However, Ni can also be measured in urine, feces, serum, and hair, which are the biomarkers of choice to assess exposure and internal dose [307]. High correlations between exposure to Ni and urine and serum Ni concentrations have been reported in several occupational studies [308–311]. Serum and urine Ni reflect short-term exposures, over the past 24–48 h [307]. Population levels of Ni in serum and urine are estimated to range from 0.2 and 1–3 μg/L, respectively. Whole blood is not a good biomarker of Ni [312]. Ni can accumulate in the placental tissue [313]. However, to our knowledge, no studies have investigated relationships between placental concentrations of Ni and more established biomarkers.
Tin
Major Sources of Exposure
Tin can be found in its divalent (Sn 2 +) or tetravalent form (Sn + 4) in soil, rocks, and water. Tin is used in several industrial activities in its inorganic form, including the production of glass, paints, perfumes and cosmetics production and food packages. Tin may also form organic compounds, such as tributyltin or triphenyltin, among others [314]. Organotin compounds are used as heat stabilizers, agrochemicals, and antifouling paints [314, 315]. The use of tributyltin in boat paints is a contamination source for marine ecosystems and its potential bioaccumulation in fish could be a source of exposure to Sn in the diet [315]. Tin is used in e-cigarette soldered joints and has been documented in e-cigarette aerosols [293, 316]. The main routes of exposure to Sn are oral ingestion, inhalation, and dermal contact [314, 317]. In the general population, ingestion of contaminated water [318], food, and beverages [319, 320] is the major source of exposure. Tin-lined cans, widely used in food packages, constitute a relevant dietary source. Populations with higher canned food consumption have a higher body burden of inorganic Sn [314, 321]. Similarly, marine food contributes to a relevant burden of exposure to organic Sn, as high levels of these compounds have been reported in the blood of individuals with high fish intake [322, 323]. It is estimated that ~ 90% of the US population has detectable levels of Sn in urine [324]. Dermal contact is most relevant in occupational settings [314].
Absorption, Biotransformation, and Elimination
After absorption, the majority of Sn is distributed from the blood stream into soft tissues and bone. The half-life of Sn in blood and soft tissue ranges from 1 to 3 days in animal models [314], compared with 2–3 months for bone [314].
The main route of elimination for both inorganic and organic Sn is urine [314], although a biliary route of elimination has been described as well [325]. There is limited data on the absorption kinetics, mechanisms of action, and measurement of Sn body burden in humans.
Biomarkers
Tin levels can be estimated in blood, urine, soft tissues, and bone. Inorganic Sn is usually measured in biological specimens, as the measurement of organic Sn requires sampling speciation for specific organic forms (e.g., alkyltin compounds) and their metabolites. Blood Sn is a biomarker of recent exposure to inorganic Sn within the previous 2 to 15 days [314]. Inorganic Sn accumulates in bone for an estimated period of 2–3 months [314]. However, only invasive techniques are available for measuring bone Sn, which limits its practical applicability. Urinary measurement of both inorganic and organic Sn is a good biomarker of exposure, as urine is the main route of elimination. Urine samples can be digested with acid to eliminate organic matter and then oxidized to extract Sn(+ 4). Measurements of inorganic Sn in urine can be used as a marker of exposure in human studies [326]. The collection of urine in plastic tubes compared to glass vials can reduce the ability of measuring organic Sn species by 70% [326].
Uranium
There are three main forms of U: natural U, enriched U, and depleted U [327, 328]. Their chemical properties, however, including chemical toxicity, are identical [328]. Radioactivity in U takes thousands of years to naturally decay, which gives this element an extremely long halflife [328]. For this section, we will focus on the chemical toxicity of natural U, as radiation effects are outside the scope of this review.
Major Sources of Exposure
Uranium is a component of rocks and soil and can occur naturally in groundwater. Uranium can also undergo oxidation–reduction reactions and combine with organic compounds in the environment. The mining of U-containing rocks for nuclear fuel and the production of phosphate fertilizers, which are also a source of Cd [329, 330], are the main anthropogenic sources of U [331–335]. These activities can result in contamination of soil, water, and air with U [334]. The main routes of exposure to U in the general population are through drinking water and diet [336]. Drinking water contamination with U is particularly relevant in the Western US, where the leaching from abandoned mines into the water systems disproportionately affects American Indian communities [337–339].
Uranium can be adsorbed from the soil into the roots of plants, while the reported bioaccumulation rates in plants and fish are low, tubercules and root vegetables can adsorb U on its surface [340]. The daily dietary intake of U is estimated to range between 1 and 20 μg [341, 342]. Dermal absorption and inhalation [343, 344] absorption can occur as well. Inhalation is the main route of exposure in occupational settings such as mines, and dermal exposure is notable in military personal [345]. Occupational exposure can occur in workers involved in U mining and milling and individuals working with U weapons and phosphate fertilizers, as well as populations working and living close to fossil fuel plants [336].
Absorption, Biotransformation, and Elimination
The toxicity of U depends on its solubility, particle size, and exposure duration [346]. Water-soluble U compounds (e.g., hexavalent U, uranyl nitrate, uranyl fluoride) are more toxic than those with low solubility (e.g., tetravalent U, sodium diuranate). Insoluble U compounds mostly exhibit toxicity when they are inhaled directly into the lungs, where they can accumulate [328].
Bioavailability and absorption are low in all routes of exposure. The absorption in the GI tract ranges from 0.1 to 6%, depending on the solubility of the ingested compound [347]. The absorption rate through inhalation is also low and ranges from 0.2 to 5% [348]. Overall, previous population studies have documented an absorption rate of less than 2% [349–351]. Fasting status and low-iron diet have been shown to increase absorption [347]. Some studies hypothesize that the DMT1 transporter may be involved in U absorption in the GI tract [347], but no studies have demonstrated this.
From the bloodstream, U is mostly distributed into bone, kidneys, and liver [331], with bone tissue accumulating most of the body burden [352, 353]. In animal models, U has been shown to cross the placenta [327]. The half-life of U is estimated to be 70–200 days in the bone and between 2 to 6 days in the kidney. Uranium usually undergoes some metabolic transformations, including the oxidation of tetravalent U to hexavalent U then a uranyl ion. Uranium compounds have high affinity for proteins in plasma, mostly transferrin, and the proximal tubule where they tend to bind [354–357]. For absorbed U, urine is the main route of elimination; it is estimated that more than 70% of the body U is filtered by the kidney [342], which is the main target organ for toxicity [358, 359]. A study in veterans exposed to high doses of U was able to document U in urine up to 7 years after exposure concluded [360]. Other elimination routes include feces, sweat, and exhaled breath. The main route of elimination for non-absorbed U is feces.
Biomarkers
Uranium can be measured in urine, feces, nails, hair [361–364], bone, and soft tissues. Total urinary U, including the determination of U isotopes, is the biomarker of choice for exposure [328]. Urine U is considered a good biomarker from all sources of exposure, as kidney filtration is the main route of elimination for this chemical [346, 354, 365–371]. Urinary U is representative of exposure over the past 2 weeks, one spot measurement can be used as an indicator of chronic exposure in populations with constant exposures [360, 372–374]. Previous studies have reported a high correlation between urine U and environmental U in air, water, and food in chronically exposed populations [375]. For every 1 μg/dL intake of U, an increase of 2.06 ng/dL, 37 ng/g, and 0.05 ng/g of this compound is expected in urine, hair, and nails respectively [363]. Hair and nail U are positively correlated, and increasing evidence suggests that both may reflect exposure from the previous weeks to months; however, more studies are needed to establish these measures as biomarkers of exposure [376]. The determination of hair U requires sample decontamination [364]. Uranium also be measured in bone and soft tissues, as it distributes within these organs in the body; previous studies have reported a significant association between water and bone U in adult men [377]. However, noninvasive methods for the assessment of U in bone tissue are not available, which is a major limitation [377].
Essential Metals
Table 2 includes a summary of the most frequently used biomarkers, sample sources, their half-lives, and other considerations for the essential metals included in this review. Essential metals have several physiological functions in the human body. Essential metals are tightly regulated in the body by metabolic mechanisms to guarantee their availability for cellular functions. Both low and high levels of essential metals are associated with adverse health effects. However, while the health impacts of essential metal deficiencies are well characterized, there is still a need to further assess the impact of excessive levels of essential metals on human health.
Table 2. Summary of the most commonly used biomarkers for essential metals, their timing and important considerations when including in a study.
Timing is divided into 4 categories according to the half-lives of the biomarkers in the body: Acute (1–3 days), Recent (up to 1 month), Sub-Chronic (1 to 6 months), Chronic (>6 months).
| Essential Metal | Biomarker | Timing | Established Biomarker | Considerations |
|---|---|---|---|---|
| Manganese | Whole Blood | Acute | Yes | Limited sensitivity Good marker in occupational studies |
| Urinea | Unclear | No | Limited validity, urine is a minor elimination pathway | |
| Hairb | Sub-chronic | Yes | Variability with hair color | |
| Toenail/Fingernailb | Sub-chronic | Unclear | Similar to hair manganese | |
| Teeth | Chronic | Unclear | Correlates well with bone and blood manganese | |
| Exhaled Breath | Acute | Unclear | Occupational studies only | |
| Brain | Chronic | Unclear | Requires MRI | |
| Molybdenum | Whole Blood | Unclear | No | Window of exposure relfected is unclear |
| Urinea | Recent | Yes | Sensitive to changes in dietary intake | |
| Hairb | Unclear | No | Window of exposure relfected is unclear | |
| Toenail/Fingernailb | Unclear | No | Window of exposure relfected is unclear | |
| Selenium | Whole Blood | Acute/Sub-chronic | Yes | Tightly regulated levels |
| Red Blood Cells | Sub-chronic | Yes | Tightly regulated levels | |
| Plasma | Acute | Yes | Decreases with smoking, malnutrition, older age, inflammatory processes | |
| Selenoprotein P in plasma | Sub-Chronic | Unclear | Heterogeneous findings | |
| Urinea | Recent | Yes | Integrates selenosugars and inorganic selenium | |
| Hairb | Unclear | No | Hair decontamination is very difficult | |
| Toenail/Fingernailb | Chronic | Yes | Good correlation with dietary intake | |
| Placenta | Unclear | Unclear | Emerging biomarker | |
| Zinc | Whole Blood | Acute | Tightly regulated levels | |
| Plasma | Acute | Yes | Low sensitivity and specificity, high variability when plasma expansion (e.g., pregnancy) 0.1% of the body burden |
|
| Urinea | Acute | Unclear | Sensitive to Zn supplementation but less sensitive to Zn depletion Influenced by conditions that increase protein catabolism such as strenuous exercise and diabetes Changes during pregnancy and lactation | |
| Hairb | Unclear | No | Window of exposure relfected is unclear | |
| Toenail/Fingernailb | Sub-Chronic | Unclear | Subject to seasonal variations |
Needs adjustment for urine dilution (creatinine or specific gravity)
Sample requires environmental decontamination
Manganese
Manganese is an essential element, which plays important roles in bone and cartilage formation as well as wound healing [378, 379]. At the molecular level, Mn is a cofactor for several enzymes (e.g., glutamine synthetase) and is involved in developmental, reproductive, immune, and energy production processes including glucose metabolism and mitochondrial function [380]. However, at elevated levels, Mn can exhibit neurotoxic effects [381].
Major Sources of Exposure
Manganese is usually found in combination with other elements, forming oxides, carbonates, or silicates [382]. The most frequent metallic forms are Mn (+ 2) and Mn (+ 3) [380]. Due to its chemical properties, Mn is used in several industrial processes including batteries, steel, cosmetics, leather, glass, and ceramics manufacturing. Gas and fossil fuel combustion can lead to the emission of Mn phosphate [383]. This compound is also used as an additive to pesticides and fungicides in agriculture. Recently, it has been proposed as a “less toxic” alternative contrast agent to gadolinium in medical procedures such as magnetic resonance imaging (MRI) [384, 385], and it is commonly added to parenteral nutrition preparations for children [386]. The main route of exposure to Mn in the general population is ingestion through diet and water. The mean daily Mn intake in the diet is estimated to range between 0.7 to 10.9 mg/day [383, 387]. Foods with high-Mn concentrations include rice, nuts, whole grains, and legumes. Tea, chocolate, and some seafoods have high levels of Mn as well. The mean intake of Mn from drinking water is estimated to be around 0.02 mg/day [387]. However, some populations, such as private well users in the US, may be exposed to higher levels [388]. Inhalation can be the main route of exposure to Mn in the occupational setting [380], particularly for miners, welders, and smelter workers [389, 390].
Absorption, Biotransformation, and Elimination
The absorption rate of Mn in the GI tract is estimated to range from 3 to 5% [391]. Mn can be absorbed by passive diffusion or by transporters including DMT1, which is used by other divalent cations, such as iron and calcium [392]. As a result, higher Mn absorption rates have been documented in iron-deficient populations due to the overexpression of DMT1 transporters [393, 394]. Absorption rates are higher in women compared with men, which may be influenced by iron status [395], and in children compared with adults [396]. Similar to iron, high dietary calcium intake has an antagonistic effect on Mn absorption [397].
Inhaled Mn can enter the bloodstream from the lungs or be transported directly from the olfactory bulb into the brain [398, 398–400]. From the bloodstream, Mn is distributed and accumulated in the liver, the pancreas, the bone tissue, the kidneys, and the brain [401–403]. Blood Mn levels are tightly regulated. Mn has a high affinity for proteins, including albumin and transferrin [404], as well as transporters such as DMT1, found in the red blood cells [405]. Endogenously, Mn is mostly found in its divalent and trivalent oxidation forms, which can undergo oxidation and reduction reactions. The liver is responsible for Mn conjugation into the bile, which leads to its excretion in the feces, the main route of elimination [380, 391]. Mn can also be eliminated in urine and sweat [406] and has been documented in human milk [407–411]. Studies using animal models have reported that the half-life of Mn ranges from 5 to 74 days in the brain and up to 690 days in bone [412–414]. For humans, the estimated average half-life of Mn for bone is 9 years [415]. Mn can accumulate and cross the placenta, and both high and low Mn exposures during the prenatal period have been associated with adverse health outcomes and DNA methylation changes in the offspring [416, 417].
Biomarkers
Urine and blood are the most frequently used biomarkers of Mn exposure. However, they present several limitations. Studies examining the correlation between environmental Mn and its concentrations in blood and urine have been heterogeneous. While some occupational studies found high correlations between dust or airborne Mn with blood Mn in groups of workers [400, 408, 418–421], findings among non-occupationally exposed individuals have been inconclusive [408, 419, 420, 422, 423]. Mn in the bloodstream is rapidly cleared through the liver and excreted into the bile and therefore has a short half-life in blood [424]. Blood Mn has a high intra-individual variability, which limits its sensitivity as a biomarker. As urine is a minor elimination pathway for Mn, its usefulness as a biomarker of exposure is limited [425–427]. Mn can also be assessed in other biological assays including feces, hair, nails, saliva, teeth, bone, and cerebrospinal fluid. Hair Mn correlates well (r = 0.48) with environmental Mn exposure from drinking water [428]. It has been proposed as a biomarker of exposure within the previous months and it is considered the best biomarker of exposure in children [428–431]. However, hair requires decontamination, and Mn concentrations can vary with hair color, as Mn has a high affinity for pigments [432]. Water Mn at concentrations exceeding 9.8 μg/L has a moderate correlation with toenail Mn (r = 0.38), suggesting the possible use of nail Mn as a biomarker of moderate to high Mn exposure [433]. Imaging diagnostic techniques such as MRI also allows for the identification of Mn accumulation in the brain [434]. Animal models have shown a high correlation between Mn concentrations in teeth and blood (r = 0.69, p < 0.001) and also between teeth and bone (r = 0.76, p < 0.001) [432].
Molybdenum
Major Sources of Exposure
Molybdenum is an essential micronutrient for plants and animals that is widely present in soil and water, but can also be found in the atmosphere. It is typically present as a mineral forming molybdenite, ferrimolybdenite, and jordisite [435, 436], and as isopolyanions such as molybdate, which form Mo salts. Mo has 5 oxidation states, with Mo (IV) and Mo (VI) being the most common. The environmental pH has an influence on this oxidation state, which determines Mo bioavailability and soil adsorption [437, 438]. Mo is widely used in industrial and mining activities, including metallurgical applications, followed by its use as a catalyst and as a pigment [439]. Some fertilizers also contain Mo salts [440]. Typically, it is used in combination with other metals such as chromium, Ni, Mn, tungsten, and cobalt, among others [441]. A radioactive isotope of Mo (99Mo) is used in nuclear medicine to conduct imaging scans [442] and can be a source of exposure to ionizing radiation in the hospital setting [435].
For the general population, the main route of exposure to Mn is ingestion from the diet. Leafy vegetables, grains, legumes such as beans, animal organs, and milk constitute the main sources of Mo in the diet [443]. Exposure from drinking water and air is relevant in mining and industrial occupational settings, as well as for populations living near contaminated areas [441]. Mo has also been found to cooccur with As and tungsten in groundwater samples in Bangladesh [388, 444].
Biotransformation/elimination
The absorption of Mo in the GI tract is variable and strongly depends on the dose and its ingestion with other compounds. When ingested with food, its bioavailability ranges from 20 to 60% [445]. Nevertheless, it goes up to 80–100% in experimental studies when stable isotopes were administered orally and intravenously to healthy volunteers [446–448]. Its transportation through the GI tract mucosa has not yet been elucidated, but anion transporters may be involved, according to animal models [446–448]. The absorption of inhaled Mo depends on particle size and solubility. Large particles are deposited in the bronchi and transported into the GI tract, and small particles can be directly absorbed from the alveoli into the bloodstream. Hexavalent Mo is the most soluble compound [436]. From the bloodstream, Mo is mostly distributed to the liver and the kidney, its main organs of accumulation [88, 449–455]. Mo can cross the placenta and be distributed to the fetus during pregnancy and it has been documented in human milk [456]. Mo can undergo oxidation and reduction processes after absorption. Physiologically, Mo in its molybdate oxidation form is incorporated into the cell where it binds to a molybdopterin and forms the enzymatic cofactor Moco (a sulfur-molybdate complex) [457–459], which plays a role in cell metabolism [460]. The main route of Mo elimination is through the kidney. Urine concentrates up to 90% of the absorbed dose of Mo from all routes of exposure. The presence of copper and sulfates accelerates Mo elimination by inhibiting its tubular reabsorption [461]. Biliary excretion is the second major route of elimination [457].
Biomarkers
Molybdenum can be measured in urine, blood, plasma, hair, and some tissues. Given that urine is the main route of Mo elimination, urinary Mo is a useful biomarker of exposure and is sensitive to changes in dietary Mo intake [445, 446, 448]. Plasma Mo has also been correlated with dietary intake. However, significant changes in plasma Mo only occur at high doses (> 460 μg/day). As a result, urine Mo is the preferred biomarker for assessing short-term exposure to this metal [379]. Blood Mo increases for a few hours after dietary intake; however, because the half-life is very short, blood Mo is not an established biomarker of exposure. Hair and toenails are not reliable biomarkers of Mo exposure as there is a high inter-laboratory variability. Additionally, their correlations with blood, urine, and dietary Mo exposures are low and the window of exposure they reflect is unclear [457, 462].
Selenium
Selenium is a metalloid and an essential micronutrient for humans. It is a fundamental component of selenoproteins, a family of 25 proteins all of which require the proteinogenic amino acid selenocysteine for their functionality [463]. Selenoproteins play a role in thyroid hormone metabolism, the immune system, antioxidant defense processes (e.g., glutathione peroxidase), and control of oxidation and reduction reactions intracellularly [464]. Despite its role as an essential nutrient, Se has a narrow safety margin, and excessive exposure to Se can cause adverse health effects [465, 466].
Major Sources of Exposure
Selenium is naturally found in rocks and soils and to a lesser extent in surface and groundwater [467]. Se can be found as inorganic Se mostly in soil and water in different oxidation states: as Se (+ 0), selenide (−2), which are insoluble metals, and as selenites (+ 4), and selenates (+ 6), which are soluble alkali. Organic Se compounds such as selenomethionine, selenocysteine, and dimethyl selenide are the most common sources of Se in food. The main anthropogenic sources of environmental Se are the burning of coal and fossil fuels, mining, smelting, and agricultural activities, as well as the industrial production of photocells and glass [468, 469]. The main route of Se exposure for the general population is diet, with foods such as organ meat and seafood, cereals and grains, brazil nuts, and other unshelled nuts, generally being rich in Se [465]. Some dietary supplements also contain high levels of Se [470]. Plants and aquatic organisms can bioaccumulate Se from soil and water, incorporating it into the food chain. Runoff from agriculture, industrial waste, and sewage treatment plants can directly contaminate surface and ground water sources [468]. Se bioaccumulation strongly depends on the soil concentration, environmental pH and solubility of the Se species [471, 472].
Absorption, Biotransformation, and Elimination
In the GI tract, Se has a high bioavailability with absorption ranging from 55 to 90% [473]. The absorption rate can vary across inorganic and organic Se species [474]. Selenomethionine and selenocysteine are predominantly found in animal foods [475], while selenate and selenite are predominant in fish [476], and γ-glutamyl methylselenocysteine is predominant in plants that bioaccumulate Se [477]. However, the distribution of Se species in food needs to be further studied to understand its bioavailability [473, 478]. Once in the bloodstream, Se tends to bind to proteins such as albumin but is also transported as free species by mechanisms still under research [479]. The liver is the main organ where Se undergoes metabolism and distribution, transforming into selenoproteins that are then distributed to other tissues. From plasma, Se can also be absorbed by the kidney, the testis, and the brain [480, 481]. The main route of Se elimination is urinary excretion. Under physiological conditions, excess Se is mostly transformed into monomethylated Se, which is a selenosugar, and excreted into the urine [482]. Under high exposure conditions, Se is largely transformed into di- and tri-methylated Se and excreted into urine [483].
Biomarkers
Selenium can be measured in blood, urine, hair, nails and feces. Plasma Se is the biomarker of choice for Se status, with a half-life of a few days [484]. Plasma Se decreases with age and is generally lower in smokers, people with malnutrition, and during acute inflammatory processes, reflecting poor Se status [485]. Red blood cell Se is also a good biomarker of Se with a half-life of two months [20]. Selenoprotein P (the most abundant selenoprotein) has been proposed as biomarker of exposure within the previous 2 to 4 weeks, with heterogeneous findings among studies. While some studies in healthy children and adults have reported high correlations between Selenoprotein P in plasma and Se supplementation, Selenoprotein P levels can also increase due to other factors including age, body mass index, alcohol intake, and underlying comorbidities such as fatty liver disease [486–488]. Whole blood and serum Se correlate well with dietary Se intake (r = 0.78 and 0.74, respectively) [489]. Selenium is excreted in urine as a methylated product and its methylation status depends on Se body burden. Under conditions of low exposure and within its physiological range, the main product in urine is monomethylated Se, a selenosugar. However, under higher exposure conditions, Se is mostly methylated into di- and trimethylated forms. Measurement of the different methylated products in urine has been proposed as a possible excess and body burden indicator as their proportions vary with different exposure doses [483]. Toenail Se represents a good biomarker of past exposure ranging from the prior 6 months to a year. The correlation with dietary Se intake is moderately high (p = 0.67) [489]. Hair Se, however, is subject to substantial contamination from hair products and is not considered an adequate biomarker of exposure [490, 491]. External contamination of hair Se is particularly relevant as Se is used as an active ingredient in anti-dandruff shampoo and hair growth serums [492, 493]. Maternal and cord blood Se correlate well with Se concentrations in the placenta [148, 494–496].
Zinc
Zinc is an essential nutrient [497], and is the second most abundant trace element in the body after iron [498]. Zn deficiency has been associated with impaired development [499] and deleterious effects in the central nervous system, fertility, wound healing, and immune function, among others [497]. Exposure to excess levels of Zn can have direct negative health effects on the lungs, cardiovascular, GI, and immune systems. The inhalation of Zn oxide is particularly toxic, leading to metal fume fever [500–502]. Increased Zn body burden can also interfere with the metabolism of other essential elements, including iron and copper [497].
Major Sources of Exposure
Zinc is commonly found in soil, air, surface, and groundwater, usually as a Zn oxide or forming sphalerite (ZnS) [497]. The main route of exposure to Zn in the general population is diet, with meat products and shellfish being major dietary sources of Zn [497, 503]. However, several anthropometric activities, including mining, smelting, electroplating, metallurgic operations, and the use of products that contain Zn as an additive (e.g., paints, rubber, alloys), contribute to Zn contamination [504–506]. Workers from those industries are particularly exposed to high levels of metallic Zn and Zn compounds primarily via inhalation [507]. Water and soil Zn concentrations are also high in industrial and mining areas, which constitutes an additional source of exposure for nearby populations [508].
Absorption, Biotransformation, and Elimination
Zinc can be absorbed by passive diffusion or mediated by transporters, with absorption rates of ingested Zn ranging from 20 to 30% [497]. The bioavailability of Zn in the GI tract is increased by citric acid and decreased by iron, calcium [509], phosphorus [497, 510], fiber, and phytate [511]. As a result, individuals with diets rich in vegetables have lower rates of Zn absorption. In the bloodstream, Zn binds to metalloproteins intracellularly in the red blood cells or travels in plasma, mostly bound to albumin and alpha-2 macroglobulin, which represents the active pool of Zn for metabolism [498]. Zinc is widely distributed within body tissues, with the musculoskeletal system accumulating 90% of the body burden [512], followed by the GI tract, brain, kidneys, heart, lungs, and pancreas [497, 501, 513–515]. Zinc primarily undergoes transformation and elimination in the liver. Zinc is mostly excreted in the bile [516, 517], where it can undergo enterohepatic circulation. The elimination rate in feces is 2–4 mg/ day. Urinary elimination accounts for a smaller fraction, with a net elimination rate of 0.5 mg/day [498]. Urinary excretion of Zn can increase in the presence of metabolic disorders, especially uncontrolled diabetes, in which case urinary Zn levels reflect impaired glucose metabolism rather than Zn exposure or status [518]. Zinc can also be eliminated through the skin and hair [519] and has been documented in human milk [520]. Evidence for placental Zn is very limited, although weak positive correlations have been reported between placental Zn and maternal and cord blood Zn [148].
Biomarkers
Zinc can be measured in plasma, urine, nails, hair, and other biological fluids and tissues [497]. However, Zn is mostly distributed intracellularly in the musculoskeletal system and has a slow turnover, with the functional pool of Zn remaining under 10% of the body burden [521]. As a result, the use of any single biomarker may have limited validity [498] and there is a need for the development of highly sensitive biomarkers for Zn body burden. Zinc in plasma can be used as an indicator of recent exposure but the sensitivity and specificity are low [497]. Plasma Zn tends to increase after supplementation, although findings have not been consistent in previous experimental studies [521, 522]. Plasma Zn does not represent intracellular accumulation and it is only representative of 0.1% of the body burden [523] and should be interpreted carefully in situations when there is an expansion of plasma (e.g., during pregnancy [524] or after surgery), in situations of acute Zn deficiency [525], or in the presence of other comorbidities (such as diabetes) [526]. Adequate sample collection and processing for blood Zn is fundamental for its correct interpretation, as contact with gel separators, rubber, and heparin can lead to sample contamination and hemolysis which can artificially increase plasma Zn levels [527]. Urinary Zn can be used as a biomarker of recent exposure [527]. In contrast, whole blood Zn is nota good biomarker of changes in Zn exposure or depletion because the levels of Zn in red blood cells remain stable after supplementation or depletion [528, 529]. Additionally, nails and hair are not established biomarkers of Zn exposure, as their correlations with urine and blood Zn concentrations are low and the exposure window reflected is unclear [530, 531]. Given the limitations for most Zn biomarkers, assessment of dietary Zn intakes has been proposed as the best method for estimating Zn exposure [529, 532].
Discussion
In this review, we provide up-to-date knowledge of the strengths and limitations of biomarkers for 12 metals that are frequently used in multi-metal epidemiologic analyses and discuss major sources of exposure (Fig. 1) to aid metals researchers as they plan their analyses and interpret their results.
Recommendations
Key takeaways and recommendations for biospecimens are highlighted in Figs. 2 and 3, respectively. Understanding the major pathways of metal exposure is critical for making sound analytical decisions and interpretations. For metals such as As and Hg, it is important to consider, ideally before the analysis stage, whether it is sufficient to measure total As or total Hg without speciation. For example, if the study population regularly consumes seafood, the predominant As species in blood and urine will be arsenobetaine, which is non-toxic, rather than the toxic inorganic As species [68]. Thus, biomarkers which reflect total As may not represent the exposure of interest and may lead to inappropriate interpretations of results. For some populations, measurement of speciated arsenic may even be insufficient, as some organic arsenicals are metabolized to DMA, which is also the predominant metabolite of inorganic arsenic [70], adjustment for arsenobetaine levels can be useful in these cases. Organic Hg species are also found in seafood [237–239]. Thus, if seafood consumption is common in the population, total Hg measures will primarily reflect organic Hg, which is the most toxic/neurotoxic species due to its ability to cross the blood–brain barrier, unless there is an additional source of exposure to consider. If organic Hg is of interest, and speciation is not an option, hair or toenails could be used [271–274]. Alternatively, if inorganic Hg is of interest, and seafood consumption is low in the study population, urinary Hg may be used as an appropriate biomarker [246, 250, 279]. There is no single biological matrix that is the best for all metals. For some metals, such as Pb, blood better reflects exposure than urine [20, 62, 68, 230, 246, 307, 326, 328, 361–364, 533]. However, for other metals, such as Ba and Mn, there is not yet an established biomarker that reliably reflects environmental exposure [76, 78, 108, 425–427, 534]. While most urinary markers reflect recent metal exposure, urinary Cd and Sn are exceptions, reflecting chronic exposure to these metals [130, 131]. Emerging biomarkers such as non-invasive XRF in bone allow for the assessment of chronic exposure to Pb and may help researchers overcome some of the limitations of blood Pb [208, 213–215, 225, 227]. Further, essential metals such as Mo and Zn are tightly regulated by homeostatic processes; thus, elevated levels in urine may reflect body loss and metabolic processes rather than excess exposure [445, 498]. These differences are important to keep in mind when interpreting results from multi-metal analyses.
Figure 2. Key takeaways box.
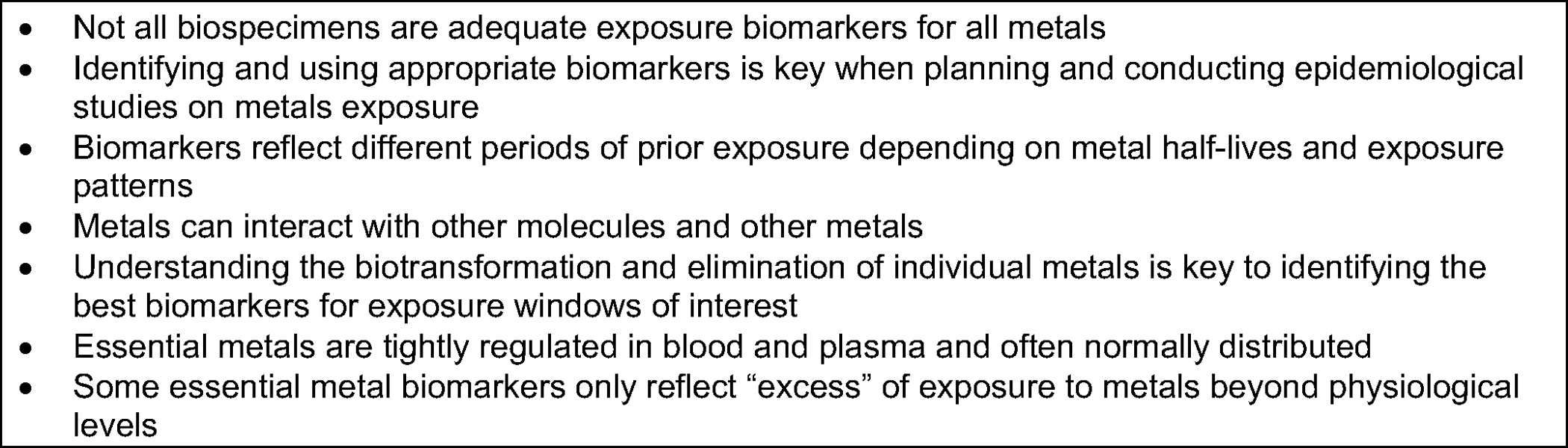
Figure 3. Key takeaways for biospecimens.

In addition to understanding metal-specific exposure sources and analytical considerations, other important considerations when using a multi-metal approach include dilution adjustment (i.e., urinary creatinine, specific gravity), and adjustment by indicators of renal function (i.e., eGFR, serum creatinine) when using urine biomarkers [535, 536]. Growth rate, matrix-dependent factors, and environmental contamination when using nails and hair, as well as potential contamination from collection tubes and storage vials and the limits of detection of the analytical procedures.
When selecting biomarkers, it is also critical to consider the exposure window of interest. Chronic exposure to metals is typically most relevant, especially for populations exposed in the low-to-moderate range, yet many biomarkers reflect recent exposures. If metal exposures vary over time (e.g., due to fluctuations in diet, changes in the environment, implementation of regulatory policies), biomarkers that reflect integrated exposures over longer durations may be preferable. Toenails are attractive in this regard, and they seem to be reliable biomarkers of exposure for As, Hg, Mn, and Se [74, 376, 462]. However, for other metals such as Cd, Pb, or Ni, toenails seem to be inadequate or need additional study [376]. Toenail growth rate may also influence toenail metal concentrations and may therefore be an additional source of measurement error and bias. It is particularly important to consider the exposure window reflected by a biomarker when the goal is to identify vulnerable windows, such as critical periods of development. For example, while many metals can be measured in cord blood and the placenta, these biospecimens can only be collected non-invasively at birth and thus typically reflect exposures which occurred in late pregnancy. The collection of maternal biospecimens during pregnancy, such as blood and urine, or the postnatal collection of biospecimens that reflect long-term exposures, such as toenails, may therefore be more appropriate for capturing earlier windows in pregnancy. Deciduous teeth also hold promise as a unique tool for identifying susceptible windows of exposure in children, as metals accumulate in primary teeth incrementally beginning in utero and can therefore be measured in distinct tooth layers, allowing for precise estimates of exposure timing [537, 538]. As a result, children’s health cohorts which did not collect biospecimens during pregnancy or in early childhood can use deciduous teeth to retrospectively reconstruct metal exposures which occurred during the pre- and postnatal periods. Adult teeth are also increasingly being used to assess the burden of exposure over the previous years.
Conclusions
As a result of the unique biotransformation and elimination pathways of different metals, not all biomarkers are adequate measures of exposure. For example, whole blood is a good biomarker of As, Cd, Pb, Hg, and Sn exposure, but it is not a good indicator for Ba, Ni, or U. For essential metals, whole blood estimates have a challenging interpretation, as these metals are tightly regulated by physiological mechanisms. Urine is not a good biomarker of Pb exposure. Hair and nails are good biomarkers of exposure to As, organic Hg, U, and essential metals such as Mn and Se. However, they are not established biomarkers for Cd, Pb, Mo, or Zn This review aims to aid metals researchers in their analysis planning, analytical approaches, and interpretation of results. As metals epidemiology moves towards embracing a multimetal/mixtures approach and expanding metal panels to include less commonly researched metals, an in-depth understanding of metal biomarkers and their limitations is key for making sound analytical decisions and appropriately interpreting results.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by National Institute of Environmental Health Sciences (NIEHS) grant P42 ES033719 (IMM, MS, ANA, TRS), NIEHS grant P30ES009089 (ANA, TRS), NIEHS grant R00ES030400 (CGH) and by a fellowship from La Caixa Foundation (ID100010434), fellowship code LCF/BQ/AA20/11820032 (IMM). The editors would like to thank Marc Weisskopf for assisting with the review of this manuscript.
Footnotes
Conflict of Interest The authors declare no competing interests.
Declarations
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Data Availability
As the manuscript is a narrative review, no data analysis was conducted and therefore, a data availability statement likely does not apply to this manuscript.
References
- 1.National Institute of Environmental Health Sciences. Human Health Exposure Analysis Resource (HHEAR). Available from. https://hhearprogram.org.
- 2.Gibson EA, Goldsmith J, Kioumourtzoglou MA. Complex Mixtures, complex analyses: an emphasis on interpretable results. Curr Environ Health Rep. 2019;6(2):53–61. 10.1007/s40572-019-00229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellavia A, James-Todd T, Williams PL. Approaches for incorporating environmental mixtures as mediators in mediation analysis. Environ Int. 2019;123:368–74. 10.1016/j.envint.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner E, Lee A, Colicino E. Environmental mixtures and children’s health: identifying appropriate statistical approaches. Curr Opin Pediatr. 2020;32(2):315–20. 10.1097/mop.0000000000000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Ten chemicals of major public health concern: World Health Organization; [updated June 1, 2020; cited 2020]. Available from: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern. [Google Scholar]
- 6.Tellez-Plaza M, Guallar E, Navas-Acien A. Environmental metals and cardiovascular disease. BMJ. 2018;362:k3435. 10.1136/bmj.k3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr SE, Bridges CC. Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci. 2017;18(5). 10.3390/ijms18051039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scammell MK, Sennett CM, Petropoulos ZE, Kamal J, Kaufman JS. Environmental and occupational exposures in kidney disease. Semin Nephrol. 2019;39(3):230–43. 10.1016/j.semnephrol.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Stammler L, Uhl A, Mayer B, Keller F. Renal effects and carcinogenicity of occupational exposure to uranium: a meta-analysis. Nephron Extrz. 2016;6(1):1–11. 10.1159/000442827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen QY, DesMarais T, Costa M. Metals and mechanisms of carcinogenesis. Annu Rev Pharmacol Toxicol. 2019;59:537–54. 10.1146/annurev-pharmtox-010818-021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White AJ, O’Brien KM, Niehoff NM, Carroll R, Sandler DP. Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology. 2019;30(1):20–8. 10.1097/ede.0000000000000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Kuang J, Zhou F, Jia Q, Lu Q, Feng C, et al. The association between prenatal cadmium exposure and birth weight: a systematic review and meta-analysis of available evidence. Environ Pollut. 2019;251:699–707. 10.1016/j.envpol.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Kim SS, Meeker JD, Carroll R, Zhao S, Mourgas MJ, Richards MJ, et al. Urinary trace metals individually and in mixtures in association with preterm birth. Environ Int. 2018;121(Pt 1):582–90. 10.1016/j.envint.2018.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claus Henn B, Ettinger AS, Hopkins MR, Jim R, Amarasiriwardena C, Christiani DC, et al. Prenatal arsenic exposure and birth outcomes among a population residing near a mining-related superfund site. Environ Health Persp. 2016;124(8):1308–15. 10.1289/ehp.1510070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah-Kulkarni S, Lee S, Jeong KS, Hong YC, Park H, Ha M, et al. Prenatal exposure to mixtures of heavy metals and neurodevelopment in infants at 6 months. Environ Res. 2020;182:109122. 10.1016/j.envres.2020.109122. [DOI] [PubMed] [Google Scholar]
- 16.Nishijo M, Nakagawa H, Suwazono Y, Nogawa K, Kido T. Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: a nested case-control analysis of a follow-up study in Japan. BMJ Open. 2017;7(7):e015694. 10.1136/bmjopen-2016-015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2015;20(1):100–20. 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou CHSJ, Harper C. Toxicological profile for arsenic. Report. Agency for toxic substances and disease registry, 2007. [PubMed] [Google Scholar]
- 20.Navas-Acien A, Tellez-Plaza M. Metals and health: science and practice. In: Boulton ML, Wallace RB, editors. Maxcy-Rosenau-Last public health & preventive medicine, 16e. New York, NY: McGraw Hill; 2022. [Google Scholar]
- 21.Wedepohl KH. The composition of the upper earth’s crust and the natural cycle of selected metals resources. Wedepohl, KH; 1984. Report No.: BIOSIS/86/36491. [Google Scholar]
- 22.Carey A-M, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, et al. Grain unloading of arsenic species in rice. Plant Physiol. 2009;152(1):309–19. 10.1104/pp.109.146126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adriano DC. Arsenic. Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. New York, NY: Springer, New York; 2001. p. 219–61. [Google Scholar]
- 24.Bencko V, Foong FYL. The history of arsenical pesticides and health risks related to the use of Agent Blue. Ann Agric Environ Med. 2017;24(2):312–6. 10.26444/aaem/74715. [DOI] [PubMed] [Google Scholar]
- 25.Garbarino JR, Bednar AJ, Rutherford DW, Beyer RS, Wershaw RL. Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environ Sci Technol. 2003;37(8):1509–14. 10.1021/es026219q. [DOI] [PubMed] [Google Scholar]
- 26.Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333(6169):134–9. 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- 27.Pacyna JM, Scholtz MT, Li Y-F. Global budget of trace metal sources. Environ Rev. 1995;3(2):145–59. 10.1139/a95-006. [DOI] [Google Scholar]
- 28.Francis CW, White GH. Leaching of toxic metals from incinerator ashes. J Water Pollut Control Fed. 1987;59(11):979–86. [Google Scholar]
- 29.Wadge A, Hutton M. The leachability and chemical speciation of selected trace elements in fly ash from coal combustion and refuse incineration. Environ Pollut. 1987;48(2):85–99. 10.1016/0269-7491(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 30.Argos M, Ahsan H, Graziano JH. Arsenic and human health: epidemiologic progress and public health implications. Rev Environ Health. 2012;27(4):191–5. 10.1515/reveh-2012-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung J-Y, Yu S-D, Hong Y-S. Environmental source of arsenic exposure. J Prev Med Public Health. 2014;47(5):253–7. 10.3961/jpmph.14.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y. Global solutions to a silent poison. Science. 2020;368(6493):818–9. 10.1126/science.abb9746. [DOI] [PubMed] [Google Scholar]
- 33.Sobel MH, Sanchez TR, Jones MR, Kaufman JD, Francesconi KA, Blaha MJ, et al. Rice intake, arsenic exposure, and subclinical cardiovascular disease among US adults in MESA. J Am Heart Assoc. 2020;9(4):e015658. 10.1161/JAHA.119.015658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Persp. 2012;120(10):1418–24. 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue J, Zartarian V, Wang S-W, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Persp. 2010;118(3):345–50. 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis MA, Signes-Pastor AJ, Argos M, Slaughter F, Pendergrast C, Punshon T, et al. Assessment of human dietary exposure to arsenic through rice. Sci Total Environ. 2017;586:1237–44. 10.1016/j.scitotenv.2017.02.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Wang W-X, Zhang L. Comparison of bioavailability and biotransformation of inorganic and organic arsenic to two marine fish. Environ Sci Technol. 2016;50(5):2413–23. 10.1021/acs.est.5b06307. [DOI] [PubMed] [Google Scholar]
- 38.Nigra Anne E, Nachman Keeve E, Love David C, Grau-Perez M, Navas-Acien A. Poultry consumption and arsenic exposure in the U.S. population. Environ Health Persp. 2017;125(3):370–7. 10.1289/EHP351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Food and Drug Administration (FDA). Arsenic-based animal drugs and poultry [updated 04/30/2021]. Available from: https://www.fda.gov/animal-veterinary/product-safety-information/arsenic-based-animal-drugs-and-poultry.
- 40.Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Brit Med Bull. 2012;104(1):175–96. 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iland HJ, Seymour JF. Role of arsenic trioxide in acute promyelocytic leukemia. Curr Treat Options Oncol. 2013;14(2):170–84. 10.1007/s11864-012-0223-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhang P On arsenic trioxide in the clinical treatment of acute promyelocytic leukemia. Leuk Res Rep. 2017;7:29–32. 10.1016/j.lrr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung JY, Yu SD, Hong YS. Environmental source of arsenic exposure. J Prev Med Public Health. 2014;47(5):253–7. 10.3961/jpmph.14.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vahter M Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–7. 10.1016/S0300-483X(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 45.Drobna Z, Styblo M, Thomas DJ. An overview of arsenic metabolism and toxicity. Curr Protoc Toxicol. 2009;42(431):4.31.1–4.31.6. 10.1002/0471140856.tx0431s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sattar A, Xie S, Hafeez MA, Wang X, Hussain HI, Iqbal Z, et al. Metabolism and toxicity of arsenicals in mammals. Environ Toxicol Pharmacol. 2016;48:214–24. 10.1016/j.etap.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Francesconi KA, Tanggaar R, McKenzie CJ, Goessler W. Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem. 2002;48(1):92–101. [PubMed] [Google Scholar]
- 48.Raml R, Goessler W, Traar P, Ochi T, Francesconi KA. Novel thioarsenic metabolites in human urine after ingestion of an arsenosugar, 2′,3′-dihydroxypropyl 5-deoxy-5-dimethylarsinoyl-beta-D-riboside. Chem Res Toxicol. 2005;18(9):1444–50. 10.1021/tx050111h. [DOI] [PubMed] [Google Scholar]
- 49.Luvonga C, Rimmer CA, Yu LL, Lee SB. Organoarsenicals in seafood: occurrence, dietary exposure, toxicity, and risk assessment considerations - a review. J Agr Food Chem. 2020;68(4):943–60. 10.1021/acs.jafc.9b07532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Research Council. Arsenic in Drinking Water: 2001 Update. Washington, DC: The National Academies Press; 2001. p. 241. [Google Scholar]
- 51.Bozack AK, Saxena R, Gamble MV. Nutritional influences on one-carbon metabolism: effects on arsenic methylation and toxicity. Annu Rev Nutr. 2018;38:401–29. 10.1146/annurev-nutr-082117-051757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall MN, Gamble MV. Nutritional manipulation of one-carbon metabolism: effects on arsenic methylation and toxicity. J Toxicol. 2012;2012:595307. 10.1155/2012/595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bozack AK, Howe CG, Hall MN, Liu X, Slavkovich V, Ilievski V, et al. Betaine and choline status modify the effects of folic acid and creatine supplementation on arsenic methylation in a randomized controlled trial of Bangladeshi adults. Eur J Nutr. 2021;60(4):1921–34. 10.1007/s00394-020-02377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bozack AK, Hall MN, Liu X, Ilievski V, Lomax-Luu AM, Parvez F, et al. Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation randomized controlled trial in Bangladesh. Am J Clin Nutri. 2019;109(2):380–91. 10.1093/ajcn/nqy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeisel SH. The supply of choline is important for fetal progenitor cells. Semin Cell Dev Biol. 2011;22(6):624–8. 10.1016/j.semcdb.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.U.S. Environmental Profection Agency. Arsenic, Inorganic. CASRN, 7440–38. Integrated risk information system (IRIS) U.S. Environmental Protection Agency; 2002. [Google Scholar]
- 57.Concha G, Vogler G, Nermell B, Vahter M. Low-level arsenic excretion in breast milk of native Andean women exposed to high levels of arsenic in the drinking water. Int Arch Occup Environ Health. 1998;71(1):42–6. 10.1007/s004200050248. [DOI] [PubMed] [Google Scholar]
- 58.Gardner RM, Nermell B, Kippler M, Grandér M, Li L, Ekström E-C, et al. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod Toxicol. 2011;31(2):210–8. 10.1016/j.reprotox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Skröder Löveborn H, Kippler M, Lu Y, Ahmed S, Kuehnelt D, Raqib R, et al. Arsenic metabolism in children differs from that in adults. Toxicol Sci. 2016;152(1):29–39. 10.1093/toxsci/kfw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamauchi H, Takata A. Arsenic metabolism differs between child and adult patients during acute arsenic poisoning. Toxicol Appl Pharmacol. 2021;410:115352. 10.1016/j.taap.2020.115352. [DOI] [PubMed] [Google Scholar]
- 61.Chowdhury UK, Rahman MM, Sengupta MK, Lodh D, Chanda CR, Roy S, et al. Pattern of excretion of arsenic compounds [arsenite, arsenate, MMA(V), DMA(V)] in urine of children compared to adults from an arsenic exposed area in Bangladesh. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2003;38(1):87–113. 10.1081/ese-120016883. [DOI] [PubMed] [Google Scholar]
- 62.Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat M-P. What is the best biomarker to assess arsenic exposure via drinking water? Environ Int. 2012;39(1):150–71. 10.1016/j.envint.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Valentine JL, Kang HK, Spivey G. Arsenic levels in human blood, urine, and hair in response to exposure via drinking water. Environ Res. 1979;20(1):24–32. 10.1016/0013-9351(79)90082-3. [DOI] [PubMed] [Google Scholar]
- 64.Concha G, Nermell B, Vahter M. Spatial and temporal variations in arsenic exposure via drinking-water in northern Argentina. J Health Popul Nutr. 2006;24(3):317–26. [PMC free article] [PubMed] [Google Scholar]
- 65.Liu T, Guo H, Xiu W, Wei C, Li X, Di Z, et al. Biomarkers of arsenic exposure in arsenic-affected areas of the Hetao Basin, Inner Mongolia. The Science of the total environment. 2017;609:524–34. 10.1016/j.scitotenv.2017.07.120. [DOI] [PubMed] [Google Scholar]
- 66.Buchet JP, Lauwerys R, Roels H. Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int Arch Occ Env Hea. 1981;48(2):111–8. 10.1007/BF00378431. [DOI] [PubMed] [Google Scholar]
- 67.Gomez-Caminero A, Howe PD, Hughes M, Kenyon E, Lewis D, Moore M, et al. Arsenic and arsenic compounds: World Health Organization. 2001. [Google Scholar]
- 68.Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111(1):110–8. 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111(1):110–8. 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, et al. Estimation of inorganic arsenic exposure in populations with frequent seafood intake: evidence from MESA and NHANES. Am J Epidemiol. 2016;184(8):590–602. 10.1093/aje/kww097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grau-Perez M, Navas-Acien A, Galan-Chilet I, Briongos-Figuero LS, Morchon-Simon D, Bermudez JD, et al. Arsenic exposure, diabetes-related genes and diabetes prevalence in a general population from Spain. Environ Pollut. 2018;235:948–55. 10.1016/j.envpol.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall M, Gamble M, Slavkovich V, Liu X, Levy D, Cheng Z, et al. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Persp. 2007;115(10):1503–9. 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katz SA. On the use of hair analysis for assessing arsenic intoxication. Int J Environ Res Pu. 2019;16(6):977. 10.3390/ijerph16060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Signes-Pastor AJ, Gutiérrez-González E, García-Villarino M, Rodríguez-Cabrera FD, López-Moreno JJ, Varea-Jiménez E, et al. Toenails as a biomarker of exposure to arsenic: a review. Environ Res. 2021;195:110286. 10.1016/j.envres.2020.110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Punshon T, Davis MA, Marsit CJ, Theiler SK, Baker ER, Jackson BP, et al. Placental arsenic concentrations in relation to both maternal and infant biomarkers of exposure in a US cohort. J Expo Sci Env Epid. 2015;25(6):599–603. 10.1038/jes.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moffett D, Smith-Simon C, Stevens Y-W. Toxicological profile for barium and barium compounds. Report. Agency for Toxic Substances and Disease Registry; 2007. [PubMed] [Google Scholar]
- 77.Klaassen CD, Watkins JB, Casarett LJ. Casarett & Doull’s essentials of toxicology. New York: McGraw-Hill Medical; 2010. [Google Scholar]
- 78.Kravchenko J, Darrah TH, Miller RK, Lyerly HK, Vengosh A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Hlth. 2014;36(4):797–814. 10.1007/s10653-014-9622-7. [DOI] [PubMed] [Google Scholar]
- 79.Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M. Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Current Environ Health Rep. 2016;3(4):416–33. 10.1007/s40572-016-0117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choudhury H, Cary R, World Health Organization, International Programme on Chemical Safety. Barium and barium compounds. Geneva: World Health Organization; 2001. [Google Scholar]
- 81.Environmental Protection Agency US. Proceedings of the technical workshops for the hydraulic fracturing study: chemical & analytical methods. Washington, D.C.: Office of Research and Development, U.S. Environmental Protection Agency; 2011. [Google Scholar]
- 82.Centers for Disease Control and Prevention. Fourth national report on human exposure to environmental chemicals, 2009. In: Services DoHaH, editor. 2009. [Google Scholar]
- 83.Meister RT, Sine C. Crop protection handbook, Volume 100. Willoughby, Ohio: Meister Media Worldwide. 2014. [Google Scholar]
- 84.Kenig J, Richter P, Żanowska K. Barium enema in the treatment algorithm of lower gastrointestinal tract bleeding. Pol Przegl Chir. 2013;85(8):467–70. 10.2478/pjs-2013-0072. [DOI] [PubMed] [Google Scholar]
- 85.Puac P, Rodríguez A, Vallejo C, Zamora CA, Castillo M. Safety of contrast material use during pregnancy and lactation. Magn Reson Imaging C. 2017;25(4):787–97. 10.1016/j.mric.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Peana M, Medici S, Dadar M, Zoroddu MA, Pelucelli A, Chasapis CT, et al. Environmental barium: potential exposure and health-hazards. Arch Toxicol. 2021;95(8):2605–12. 10.1007/s00204-021-03049-5. [DOI] [PubMed] [Google Scholar]
- 87.Calabrese EJ, Canada AT, Sacco C. Trace elements and public health. Annu Rev Publ Health. 1985;6(1):131–46. [DOI] [PubMed] [Google Scholar]
- 88.Schroeder HA, Tipton IH, Nason AP. Trace metals in man: strontium and barium. J Chron Dis. 1972;25(9):491–517. 10.1016/0021-9681(72)90150-6. [DOI] [PubMed] [Google Scholar]
- 89.Underwood E. Trace elements in human and animal nutrition: Elsevier. 2012. [Google Scholar]
- 90.Karlsson H, Toprak M, Fadeel B, Nordberg G, Fowler B, Nordberg M. Handbook on the toxicology of metals. Cambridge: Academic Press; 2014. [Google Scholar]
- 91.Brenniman G, Levy P. Epidemiological study of barium in Illinois drinking water supplies. Adv Mod Env. 1984;9:231–49. [Google Scholar]
- 92.Oskarsson A. Chapter 29 - Barium. In: Nordberg GF, Fowler BA, Nordberg M, editors. Handbook on the toxicology of metals. 4th ed. San Diego: Academic Press; 2015. p. 625–34. [Google Scholar]
- 93.Foster S, Choudhury H, Colman J, Ingerman L, Robbins P. Barium and compounds CASRN 7440–39-3 |IRIS|US EPA, ORD. Integrated risk information system (IRIS) U.S. Environmental Profection Agency 2005. [Google Scholar]
- 94.Sutton A, Humphreys ER, Shepherd H, Howells GR. Reduction in the retention of radioactive barium in rats following the addition of sodium alginate derivatives to the diet. Int J Radiat Biol Relat Stud Phys Chem Med. 1972;22(3):297–300. 10.1080/09553007214551081. [DOI] [PubMed] [Google Scholar]
- 95.Lengemann F The site of action of lactose in the enhancement of calcium utilization. J Nutr. 1959;69:23–7. [DOI] [PubMed] [Google Scholar]
- 96.Berggren P-O, Andersson T, Hellman B. The interaction between barium and calcium in β-cell-rich pancreatic islets. Biomed Res. 1983;4(2):129–38. [Google Scholar]
- 97.Harrison GE, Carr TEF, Sutton A, Rundo J. Plasma concentration and excretion of calcium-47, strontium-85, barium-133 and radium-223 following successive intravenous doses to a healthy man. Nature. 1966;209(5022):526–7. 10.1038/209526b0. [DOI] [PubMed] [Google Scholar]
- 98.Edel J, Di Nucci A, Sabbioni E, Manzo L, Tonini M, Minnoia C, et al. Biliary excretion of barium in the rat. Biol Trace Elem Res. 1991;30(3):267–76. 10.1007/BF02991421. [DOI] [PubMed] [Google Scholar]
- 99.Bhoelan BS, Stevering CH, van der Boog ATJ, van der Heyden MAG. Barium toxicity and the role of the potassium inward rectifier current. Clin Toxicol. 2014;52(6):584–93. 10.3109/15563650.2014.923903. [DOI] [PubMed] [Google Scholar]
- 100.Foster PR, Elharrar V, Zipes DP. Accelerated ventricular escapes induced in the intact dog by barium, strontium and calcium. J Pharmacol Exp Ther. 1977;200(2):373–83. [PubMed] [Google Scholar]
- 101.Jaklinski AMJ, Przegalinski E. Experimental studies on barium poisoning. J Forensic Med. 1967;14:13–5. [Google Scholar]
- 102.Schott GD, McArdle B. Barium-induced skeletal muscle paralysis in the rat, and its relationship to human familial periodic paralysis. J Neurol Neurosurg Psychiatry. 1974;37(1):32–9. 10.1136/jnnp.37.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention. National report on human exposure to environmental chemicals. Atlanta, GA: CDC; 2001. [Google Scholar]
- 104.Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assists environmental medicine physicians. Altern Med Rev. 2010;15(2). [PubMed] [Google Scholar]
- 105.Mauras Y, Allain P. Determination of barium in water and biological fluids by emission spectrometry with an inductively-coupled plasma. Anal Chim Acta. 1979;110(2):271–7. [Google Scholar]
- 106.Schramel P. ICP and DCP emission spectrometry for trace element analysis in biomedical and environmental samples. A review. Spectrochim Acta B. 1988;43(8):881–96. 10.1016/0584-8547(88)80194-0. [DOI] [Google Scholar]
- 107.Shiraishi K, Kawamura H, Tanaka G-I. Determination of alkaline-earth metals in foetus bones by inductively-coupled plasma atomic-emission spectrometry. Talanta. 1987;34(10):823–7. [DOI] [PubMed] [Google Scholar]
- 108.Kato M, Ohgami N, Ohnuma S, Hashimoto K, Tazaki A, Xu H, et al. Multidisciplinary approach to assess the toxicities of arsenic and barium in drinking water. Environ Health Prev Med. 2020;25. 10.1186/s12199-020-00855-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tabelin CB, Igarashi T, Villacorte-Tabelin M, Park I, Opiso EM, Ito M, et al. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: a review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci Total Environ. 2018;645:1522–53. 10.1016/j.scitotenv.2018.07.103. [DOI] [PubMed] [Google Scholar]
- 110.Turner A Cadmium pigments in consumer products and their health risks. Sci Total Environ. 2019;657:1409–18. 10.1016/j.scitotenv.2018.12.096. [DOI] [PubMed] [Google Scholar]
- 111.Ashizawa A, Faroon O, Ingerman L, Jenkins K, Tucker P, Wright S. Toxicological profile for cadmium. Report. Agency for Toxic Substances and Disease Registry; 2012. [PubMed] [Google Scholar]
- 112.Staessen JA, Vyncke G, Lauwerys RR, Roels HA, Celis HG, Claeys F, et al. Transfer of cadmium from a sandy acidic soil to man: a population study. Environ Res. 1992;58(1–2):25–34. [DOI] [PubMed] [Google Scholar]
- 113.Lalor GC. Review of cadmium transfers from soil to humans and its health effects in the Jamaican environment. Sci Total Environ. 2008;400(1–3):162–72. [DOI] [PubMed] [Google Scholar]
- 114.Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut R. 2016;23(9):8244–59. [DOI] [PubMed] [Google Scholar]
- 115.Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environ Health Persp. 2012;120(2):204–9. 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olmedo P, Grau-Perez M, Fretts A, Tellez-Plaza M, Gil F, Yeh F, et al. Dietary determinants of cadmium exposure in the Strong Heart Family Study. Food Chem Toxicol. 2017;100:239–46. 10.1016/j.fct.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choudhury H, Harvey T, Thayer WC, Lockwood TF, Stiteler WM, Goodrum PE, et al. Urinary cadmium elimination as a biomarker of exposure for evaluating a cadmium dietary exposure-biokinetics model. J Tox Env Health A. 2001;63(5):321–50. [DOI] [PubMed] [Google Scholar]
- 118.U.S. Environmental Protection Agency. Second integrated urban air toxics report to congress [updated 11/24/2020]. Available from: https://www.epa.gov/urban-air-toxics/second-integrated-urban-air-toxics-report-congress
- 119.Otahara Y, Izuno T, Tatemichi M, Sugita M. Estimating cadmium absorption rate in digestive organs calculated from information of studies on cadmium conducted in Japan. J UOEH. 2003;25(2):171–83. 10.7888/juoeh.25.171. [DOI] [PubMed] [Google Scholar]
- 120.McLellan J, Flanagan P, Chamberlain M, Valberg L. Measurement of dietary cadmium absorption in humans. J Tox Env Health A. 1978;4(1):131–8. [DOI] [PubMed] [Google Scholar]
- 121.Rentschler G, Kippler M, Axmon A, Raqib R, Ekström E-C, Skerfving S, et al. Polymorphisms in iron homeostasis genes and urinary cadmium concentrations among nonsmoking women in Argentina and Bangladesh. Environ Health Persp. 2013;121(4):467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nishijo M, Satarug S, Honda R, Tsuritani I, Aoshima K. The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol Cell Biochem. 2004;255(1– 2):87–92. 10.1023/b:mcbi.0000007264.37170.39. [DOI] [PubMed] [Google Scholar]
- 123.Yu H-t, Zhen J, Leng J-y, Cai, Ji H-l, Keller BB. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol Sin. 2020:1–7. 10.1038/s41401-020-0396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grau-Perez M, Voruganti VS, Balakrishnan P, Haack K, Goessler W, Franceschini N, et al. Genetic variation and urine cadmium levels: ABCC1 effects in the Strong Heart Family Study. Environ Pollut. 2021;276:116717. 10.1016/j.envpol.2021.116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. The effects of cadmium toxicity. Int J Environ Res Public Health 2020;17(11). 10.3390/ijerph17113782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Järup L, Berglund M, Elinder CG, Nordberg G, Vanter M. Health effects of cadmium exposure – a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24:1–51. [PubMed] [Google Scholar]
- 127.Prozialeck WC, Edwards JR. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for bio-monitoring and therapeutic interventions. J Pharmacol Exp Ther. 2012;343(1):2–12. Epub 2012/06/07. 10.1124/jpet.110.166769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. 2017;28(10):2838–44. 10.1681/asn.2017040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kjellström T, Nordberg GF. A kinetic model of cadmium metabolism in the human being. Environ Res. 1978;16(1):248–69. 10.1016/0013-9351(78)90160-3. [DOI] [PubMed] [Google Scholar]
- 130.Hsieh CY, Wang SL, Fadrowski JJ, Navas-Acien A, Kuo CC. Urinary concentration correction methods for arsenic, cadmium, and mercury: a systematic review of practice-based evidence. Curr Environ Health Rep. 2019;6(3):188–99. 10.1007/s40572-019-00242-8. [DOI] [PubMed] [Google Scholar]
- 131.Vacchi-Suzzi C, Kruse D, Harrington J, Levine K, Meliker JR. Is urinary cadmium a biomarker of long-term exposure in humans? a review. Curr Environ Health Rep. 2016;3(4):450–8. 10.1007/s40572-016-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vacchi-Suzzi C, Porucznik CA, Cox KJ, Zhao Y, Ahn H, Harrington JM, et al. Temporal variability of urinary cadmium in spot urine samples and first morning voids. J Expo Sci Env Epid. 2017;27(3):306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kido T, Sunaga K, Nishijo M, Nakagawa H, Kobayashi E, Nogawa K. The relation of individual cadmium concentration in urine with total cadmium intake in Kakehashi River basin. Japan Toxicol Lett. 2004;152(1):57–61. [DOI] [PubMed] [Google Scholar]
- 134.Kobayashi E, Suwazono Y, Uetani M, Inaba T, Oishi M, Kido T, et al. Association between lifetime cadmium intake and cadmium concentration in individual urine. B Environ Contam Tox. 2005;74(5):817–21. [DOI] [PubMed] [Google Scholar]
- 135.Shimbo S, Zhang Z-W, Moon C-S, Watanabe T, Nakatsuka H, Matsuda-Inoguchi N, et al. Correlation between urine and blood concentrations, and dietary intake of cadmium and lead among women in the general population of Japan. Int Arch Occ Env Hea. 2000;73(3):163–70. [DOI] [PubMed] [Google Scholar]
- 136.Meliker JR, Vacchi-Suzzi C, Harrington J, Levine K, Lui LY, Bauer DC, et al. Temporal stability of urinary cadmium in samples collected several years apart in a population of older persons. Int J Hyg Environ Health. 2019;222(2):230–4. 10.1016/j.ijheh.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ikeda M, Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, et al. Reproducibility of urinary cadmium, α 1-microglobulin, and β 2-microglobulin levels in health screening of the general population. Arch Environ Con Tox. 2004;48(1):135–40. [DOI] [PubMed] [Google Scholar]
- 138.Oliveira AS, Costa EAC, Pereira EC, Freitas MAS, Freire BM, Batista BL, et al. The applicability of fingernail lead and cadmium levels as subchronic exposure biomarkers for preschool children. Sci Total Environ. 2021;758:143583. 10.1016/j.scitotenv.2020.143583. [DOI] [PubMed] [Google Scholar]
- 139.Esteban-Vasallo MD, Aragonés N, Pollan M, López-Abente G, Perez-Gomez B. Mercury, cadmium, and lead levels in human placenta: a systematic review. Environ Health Persp. 2012;120(10):1369–77. 10.1289/ehp.1204952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Berlin M, Blanks R, Catton M, Kazantzis G, Mottet NK, Samiullah Y. Birth weight of children and cadmium accumulation in placentas of female nickel-cadmium (long-life) battery workers. IARC Sci Publ. 1992;118:257–62. [PubMed] [Google Scholar]
- 141.Lagerkvist BJ, Sandberg S, Frech W, Jin T, Nordberg GF. Is placenta a good indicator of cadmium and lead exposure? Arch Environ Health. 1996;51(5):389–94. 10.1080/00039896.1996.9934427. [DOI] [PubMed] [Google Scholar]
- 142.Falcón M, Vinas P, Osuna E, Luna A. Environmental exposures to lead and cadmium measured in human placenta. Arch Environ Health. 2002;57(6):598–602. 10.1080/00039890209602094. [DOI] [PubMed] [Google Scholar]
- 143.Kantola M, Purkunen R, Kröger P, Tooming A, Juravskaja J, Pasanen M, et al. Accumulation of cadmium, zinc, and copper in maternal blood and developmental placental tissue: differences between Finland, Estonia, and St. Petersburg Environ Res. 2000;83(1):54–66. 10.1006/enrs.1999.4043. [DOI] [PubMed] [Google Scholar]
- 144.Kippler M, Hoque AMW, Raqib R, Öhrvik H, Ekström E-C, Vahter M. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol Lett. 2010;192(2):162–8. 10.1016/j.toxlet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 145.Roels H, Hubermont G, Buchet JP, Lauwerys R. Placental transfer of lead, mercury, cadmium, and carbon monoxide in women: III. Factors influencing the accumulation of heavy metals in the placenta and the relationship between metal concentration in the placenta and in maternal and cord blood. Environ Res. 1978;16(1):236–47. 10.1016/0013-9351(78)90159-7. [DOI] [PubMed] [Google Scholar]
- 146.Ronco AM, Arguello G, Muñoz L, Gras N, Llanos M. Metals content in placentas from moderate cigarette consumers: correlation with newborn birth weight. Biometals. 2005;18(3):233–41. 10.1007/s10534-005-0583-2. [DOI] [PubMed] [Google Scholar]
- 147.Stasenko S, Bradford EM, Piasek M, Henson MC, Varnai VM, Jurasović J, et al. Metals in human placenta: focus on the effects of cadmium on steroid hormones and leptin. J Appl Toxicol. 2010;30(3):242–53. 10.1002/jat.1490. [DOI] [PubMed] [Google Scholar]
- 148.Baglan RJ, Brill AB, Schulert A, Wilson D, Larsen K, Dyer N, et al. Utility of placental tissue as an indicator of trace element exposure to adult and fetus. Environ Res. 1974;8(1):64–70. 10.1016/0013-9351(74)90063-2. [DOI] [PubMed] [Google Scholar]
- 149.Abadin H, Ashizawa A, Llados F, Stevens Y-W. Toxicological profile for lead. Report. Agency for Toxic Substances and Disease Registry; 2007. [PubMed] [Google Scholar]
- 150.U.S. Environmental Protection Agency. Prohibition on gasoline containing lead or lead additives for highway use. U.S. Environmental Protection Agency, 40 CFR part 80. 61 FR, 1996:3832–38. [Google Scholar]
- 151.Hanna-Attisha M, Lanphear B, Landrigan P. Lead poisoning in the 21st century: the silent epidemic continues. American Public Health Association; 2018:1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Environmental Protection Agency US. Model-extrapolated Estimates of airborne lead concentrations at U.S. Airports, (Office of Air Quality Planning & Standards). EPA-420-R-20–003. Research Triangle Park, NC: U.S. EPA; 2020. [Google Scholar]
- 153.World Health Organization. Children and digital dumpsites: e-waste exposure and child health. Geneva: World Health Organization; 2021. Report No.: 9240023909 Contract No.: Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 154.Brown MJ, Margolis S. Lead in drinking water and human blood lead levels in the United States. MMWR Suppl. 2012;61(4):1–9. [PubMed] [Google Scholar]
- 155.Hanna-Attisha M, LaChance J, Sadler RC, Schnepp AC. Elevated blood lead levels in children associated with the flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283–90. 10.2105/ajph.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cowell W, Ireland T, Vorhees D, Heiger-Bernays W. Ground turmeric as a source of lead exposure in the United States. Public Health Rep. 2017;132(3):289–93. 10.1177/0033354917700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Forsyth JE, Nurunnahar S, Islam SS, Baker M, Yeasmin D, Islam MS, et al. Turmeric means “yellow” in Bengali: lead chromate pigments added to turmeric threaten public health across Bangladesh. Environ Res. 2019;179(Pt A):108722. 10.1016/j.envres.2019.108722. [DOI] [PubMed] [Google Scholar]
- 158.Zartarian V, Xue J, Tornero-Velez R, Brown J. Children’s lead exposure: a multimedia modeling analysis to guide public health decision-making. Environ Health Persp. 2017;125(9):097009. 10.1289/EHP1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Centers for Disease Control and Prevention. Lead in foods, cosmetics, and medicines [updated 11/24/2020]. Available from: https://www.niehs.nih.gov/research/supported/exposure/mixtures/prime_program/index.cfm
- 160.Abt E, Fong Sam J, Gray P, Robin LP. Cadmium and lead in cocoa powder and chocolate products in the US Market. Food Addit Contam Part B Surveill. 2018;11(2):92–102. 10.1080/19393210.2017.1420700. [DOI] [PubMed] [Google Scholar]
- 161.Bornschein RL, Succop PA, Krafft KM, Clark CS, Peace B, Hammond PB. Exterior surface dust lead, interior house dust lead and childhood lead exposure in an urban environment. Trace Subst Environ Health. 1986. [Google Scholar]
- 162.Charney E, Sayre J, Coulter M. Increased lead absorption in inner city children: where does the lead come from? Pediatrics. 1980;65(2):226–31. [PubMed] [Google Scholar]
- 163.Dixon SL, Gaitens JM, Jacobs DE, Strauss W, Nagaraja J, Pivetz T, et al. Exposure of U.S. children to residential dust lead, 1999– 2004: II. The contribution of lead-contaminated dust to children’s blood lead levels. Environ Health Persp. 2009;117(3):468–74. 10.1289/ehp.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lanphear BP, Roghmann KJ. Pathways of lead exposure in urban children. Environ Res. 1997;74(1):67–73. [DOI] [PubMed] [Google Scholar]
- 165.Lanphear BP, Burgoon DA, Rust SW, Eberly S, Galke W. Environmental exposures to lead and urban children’s blood lead levels. Environ Res. 1998;76(2):120–30. [DOI] [PubMed] [Google Scholar]
- 166.Succop P, Bornschein R, Brown K, Tseng C-Y. An empirical comparison of lead exposure pathway models. Environ Health Persp. 1998;106(suppl 6):1577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Centers for Disease Control and Prevention. Blood lead reference value [updated 11/27/2020]. Available from: https://www.cdc.gov/nceh/lead/data/blood-lead-reference-value.htm
- 168.Council on Environmental Health. Prevention of childhood lead toxicity. Pediatrics. 2016;138(1). 10.1542/peds.2016-1493. [DOI] [PubMed] [Google Scholar]
- 169.Heard M, Chamberlain A. Effect of minerals and food on uptake of lead from the gastrointestinal tract in humans. Hum Toxicol. 1982;1(4):411–5. [DOI] [PubMed] [Google Scholar]
- 170.James H, Hilburn M, Blair J. Effects of meals and meal times on uptake of lead from the gastrointestinal tract in humans. Hum Toxicol. 1985;4(4):401–7. [DOI] [PubMed] [Google Scholar]
- 171.Rabinowitz MB, Kopple JD, Wetherill GW. Effect of food intake and fasting on gastrointestinal lead absorption in humans. Am J Clin Nutr. 1980;33(8):1784–8. [DOI] [PubMed] [Google Scholar]
- 172.Watson WS, Morrison J, Bethel M, Baldwin N, Lyon D, Dobson H, et al. Food iron and lead absorption in humans. Am J Clin Nutr. 1986;44(2):248–56. [DOI] [PubMed] [Google Scholar]
- 173.Blake K, Mann M. Effect of calcium and phosphorus on the gastrointestinal absorption of 203Pb in man. Environ Res. 1983;30(1):188–94. [DOI] [PubMed] [Google Scholar]
- 174.Blake K, Barbezat G, Mann M. Effect of dietary constituents on the gastrointestinal absorption of 203Pb in man. Environ Res. 1983;30(1):182–7. [DOI] [PubMed] [Google Scholar]
- 175.Maddaloni M, Lolacono N, Manton W, Blum C, Drexler J, Graziano J. Bioavailability of soilborne lead in adults, by stable isotope dilution. Environ Health Persp. 1998;106(suppl 6):1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mahaffey KR, Annest JL. Association of erythrocyte protoporphyrin with blood lead level and iron status in the Second National Health and Nutrition Examination Survey, 1976–1980. Environ Res. 1986;41(1):327–38. [DOI] [PubMed] [Google Scholar]
- 177.Marcus AH, Schwartz J. Dose—Response curves for erythrocyte protoporphyrin vs blood lead: Effects of iron status. Environ Res. 1987;44(2):221–7. [DOI] [PubMed] [Google Scholar]
- 178.de Almeida Lopes ACB, Navas-Acien A, Zamoiski R, Silbergeld EK, Carvalho MdFH, Buzzo ML, et al. Risk factors for lead exposure in adult population in southern Brazil. J Toxicol Env Heal A. 2015;78(2):92–108. [DOI] [PubMed] [Google Scholar]
- 179.de Souza ID, de Andrade AS, Dalmolin RJS. Lead-interacting proteins and their implication in lead poisoning. Crit Rev Toxicol. 2018;48(5):375–86. [DOI] [PubMed] [Google Scholar]
- 180.Jaffe EK, Volin M, Bronson-Mullins CR, Dunbrack RL Jr, Kervinen J, Martins J, et al. An artificial gene for human porphobilinogen synthase allows comparison of an allelic variation implicated in susceptibility to lead poisoning. J Biol Chem. 2000;275(4):2619–26. [DOI] [PubMed] [Google Scholar]
- 181.Rădulescu A, Lundgren S. A pharmacokinetic model of lead absorption and calcium competitive dynamics. Sci Rep. 2019;9(1):14225. 10.1038/s41598-019-50654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pemmer B, Roschger A, Wastl A, Hofstaetter JG, Wobrauschek P, Simon R, et al. Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue. Bone. 2013;57(1):184–93. 10.1016/j.bone.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aufderheide AC, Wittmers LE. Selected aspects of the spatial distribution of lead in bone. Neurotoxicology. 1992;13(4):809–19. [PubMed] [Google Scholar]
- 184.Berkowitz GS, Wolff MS, Lapinski RH, Todd AC. Prospective study of blood and tibia lead in women undergoing surgical menopause. Environ Health Persp. 2004;112(17):1673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Garrido Latorre F, Hernández-Avila M, Tamayo Orozco J, Albores Medina CA, Aro A, Palazuelos E, et al. Relationship of blood and bone lead to menopause and bone mineral density among middle-age women in Mexico City. Environ Health Persp. 2003;111(4):631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Korrick SA, Schwartz J, Tsaih S-W, Hunter DJ, Aro A, Rosner B, et al. Correlates of bone and blood lead levels among middle-aged and elderly women. Am J Epidemiol. 2002;156(4):335–43. [DOI] [PubMed] [Google Scholar]
- 187.Nash D, Magder LS, Sherwin R, Rubin RJ, Silbergeld EK. Bone density-related predictors of blood lead level among peri-and postmenopausal women in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2004;160(9):901–11. [DOI] [PubMed] [Google Scholar]
- 188.Popovic M, McNeill FE, Chettle DR, Webber CE, Lee CV, Kaye WE. Impact of occupational exposure on lead levels in women. Environ Health Persp. 2005;113(4):478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Symanski E, Hertz-Picciotto I. Blood lead levels in relation to menopause, smoking, and pregnancy history. Am J Epidemiol. 1995;141(11):1047–58. [DOI] [PubMed] [Google Scholar]
- 190.Chamberlain A, Heard M, Little P, Newton D, Wells A, Wiffen R. Investigations into lead from motor vehicles. Harwell, United Kingdom: United Kingdom Atomic Energy Authority; 1978. Report No.: AERE-R9198. [Google Scholar]
- 191.Griffin T, Coulston F, Wills H. Biological and clinical effects of continuous exposure to airborne particulate lead. Arh Hig Rada Tokisko. 1975;26(Supplement):191–208. [Google Scholar]
- 192.Hernández-Ochoa I, García-Vargas G, López-Carrillo L, Rubio-Andrade M, Morán-Martínez J, Cebrián ME, et al. Low lead environmental exposure alters semen quality and sperm chromatin condensation in northern Mexico. Reprod Toxicol. 2005;20(2):221–8. 10.1016/j.reprotox.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 193.Hursh JB, Suomela J. Absorption of 212pb from the gastrointestinal tract of man. Acta Radiol Ther Phy. 1968;7(2):108–20. 10.3109/02841866809133184. [DOI] [PubMed] [Google Scholar]
- 194.Hursh J, Schraub A, Sattler E, Hofmann H. Fate of 212Pb inhaled by human subjects. Health Phys. 1969;16(3):257–67. [DOI] [PubMed] [Google Scholar]
- 195.Kehoe RA, Suskind R, Hammond P. Studies of lead administration and elimination in adult volunteers under natural and experimentally induced conditions over extended periods of time. Food Chem Toxicol. 1987;25(6):425–93. [PubMed] [Google Scholar]
- 196.Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976;58(2):260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sears ME, Kerr KJ, Bray RI. Arsenic, cadmium, lead, and mercury in sweat: a systematic review. J Environ Public Health. 2012;2012:184745. 10.1155/2012/184745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stauber J, Florence T, Gulson B, Dale L. Percutaneous absorption of inorganic lead compounds. Sci Total Environ. 1994;145(1–2):55–70. [DOI] [PubMed] [Google Scholar]
- 199.Ettinger AS, Téllez-Rojo MM, Amarasiriwardena C, Bellinger D, Peterson K, Schwartz J, et al. Effect of breast milk lead on infant blood lead levels at 1 month of age. Environ Health Perspect. 2004;112(14):1381–5. 10.1289/ehp.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Esteban-Vasallo MD, Aragonés N, Pollan M, López-Abente G, Perez-Gomez B. Mercury, cadmium, and lead levels in human placenta: a systematic review. Environ Health Perspect. 2012;120(10):1369–77. 10.1289/ehp.1204952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Persp. 2007;115(3):455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barbosa F Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Persp. 2005;113(12):1669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Skerfving S, Bergdahl IA. Chapter 31 - Lead. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the toxicology of metals. 3rd ed. Burlington: Academic Press; 2007. p. 599–643. [Google Scholar]
- 204.Armstrong R, Chettle D, Scott MC, Somervaille LJ, Pendlington M. Repeated measurements of tibia lead concentrations by in vivo x ray fluorescence in occupational exposure. Occup Environ Med. 1992;49(1):14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Behinaein S, Chettle DR, Egden LM, McNeill FE, Norman G, Richard N, et al. The estimation of the rates of lead exchange between body compartments of smelter employees. Environ Sci-Proc Imp. 2014;16(7):1705–15. 10.1039/C4EM00032C. [DOI] [PubMed] [Google Scholar]
- 206.Chuang H-Y, Schwartz J, Tsai S-Y, Lee M-LT, Wang J-D, Hu H. Vibration perception thresholds in workers with long term exposure to lead. Occup Environ Med. 2000;57(9):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fleming D, Boulay D, Richard NS, Robin J-P, Gordon CL, Webber CE, et al. Accumulated body burden and endogenous release of lead in employees of a lead smelter. Environ Health Persp. 1997;105(2):224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gerhardsson L, Attewell R, Chettle DR, Englyst V, Lundström NG, Nordberg GF, et al. In vivo measurements of lead in bone in long-term exposed lead smelter workers. Arch Enivorn Health. 1993;48(3):147–56. 10.1080/00039896.1993.9940813. [DOI] [PubMed] [Google Scholar]
- 209.Healey N, Chettle DR, McNeill FE, Fleming DEB. Uncertainties in the relationship between tibia lead and cumulative blood lead index. Environ Health Persp. 2008;116(3):A109–A. 10.1289/ehp.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McNeill FE, Stokes L, Brito JAA, Chettle DR, Kaye WE. 109Cd K x ray fluorescence measurements of tibial lead content in young adults exposed to lead in early childhood. Occup Environ Med. 2000;57(7):465–71. 10.1136/oem.57.7.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nie LH, Wright RO, Bellinger DC, Hussain J, Amarasiriwardena C, Chettle DR, et al. Blood lead levels and cumulative blood lead index (CBLI) as predictors of late neurodevelopment in lead poisoned children. Biomarkers. 2011;16(6):517–24. 10.3109/1354750X.2011.604133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roels H, Konings J, Green S, Bradley D, Chettle D, Lauwerys R. Time-integrated blood lead concentration is a valid surrogate for estimating the cumulative lead dose assessed by tibial lead measurement. Environ Res. 1995;69(2):75–82. [DOI] [PubMed] [Google Scholar]
- 213.Specht AJ, Lin Y, Weisskopf M, Yan C, Hu H, Xu J, et al. XRF-measured bone lead (Pb) as a biomarker for Pb exposure and toxicity among children diagnosed with Pb poisoning. Biomarkers. 2016;21(4):347–52. 10.3109/1354750X.2016.1139183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Specht AJ, Weisskopf MG, Nie LH. Theoretical modeling of a portable x-ray tube based KXRF system to measure lead in bone. Physiol Meas. 2017;38(3):575–85. 10.1088/1361-6579/aa5efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Specht AJ, Dickerson AS, Weisskopf MG. Comparison of bone lead measured via portable x-ray fluorescence across and within bones. Environ Res. 2019;172:273–8. 10.1016/j.envres.2019.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Behinaein S, Chettle DR, Marro L, Malowany M, Fisher M, Fleming DEB, et al. Factors influencing uncertainties of in vivo bone lead measurement using a 109Cd K X-ray fluorescence clover leaf geometry detector system. Environ Sci-Proc Imp. 2014;16(12):2742–51. 10.1039/C4EM00446A. [DOI] [PubMed] [Google Scholar]
- 217.Lee BK, Schwartz BS, Stewart W, Ahn KD. Provocative chelation with DMSA and EDTA: evidence for differential access to lead storage sites. Occup Environ Med. 1995;52(1):13–9. 10.1136/oem.52.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee BK, Ahn KD, Lee SS, Lee GS, Kim YB, Schwartz BS. A comparison of different lead biomarkers in their associations with lead-related symptoms. Int Arch Occ Env Hea. 2000;73(5):298–304. 10.1007/s004200000132. [DOI] [PubMed] [Google Scholar]
- 219.Hoet P, Buchet J-P, Decerf L, Lavalleye B, Haufroid V, Lison D. Clinical evaluation of a lead mobilization test using the chelating agent dimercaptosuccinic acid. Clin Chem. 2006;52(1):88–96. 10.1373/clinchem.2005.051128. [DOI] [PubMed] [Google Scholar]
- 220.Bud P, Montgomery J, Evans J, Barreiro B. Human tooth enamel as a record of the comparative lead exposure of prehistoric and modern people. The Science of the total environment. 2000;263(1– 3):1–10. 10.1016/s0048-9697(00)00604-5. [DOI] [PubMed] [Google Scholar]
- 221.Asaduzzaman K, Khandaker MU, Binti Baharudin NA, Amin YBM, Farook MS, Bradley DA, et al. Heavy metals in human teeth dentine: a bio-indicator of metals exposure and environmental pollution. Chemosphere. 2017;176:221–30. 10.1016/j.chemosphere.2017.02.114. [DOI] [PubMed] [Google Scholar]
- 222.Koizumi A, Azechi M, Shirasawa K, Saito N, Saito K, Shigehara N, et al. Reconstruction of human exposure to heavy metals using synchrotron radiation microbeams in prehistoric and modern humans. Environ Health Prev Med. 2009;14(1):52–9. 10.1007/s12199-008-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Uryu T, Yoshinaga J, Yanagisawa Y, Endo M, Takahashi J. Analysis of lead in tooth enamel by laser ablation-inductively coupled plasma-mass spectrometry. Anal Sci. 2003;19(10):1413–6. 10.2116/analsci.19.1413. [DOI] [PubMed] [Google Scholar]
- 224.Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979;300(13):689–95. 10.1056/nejm197903293001301. [DOI] [PubMed] [Google Scholar]
- 225.Arora M, Kennedy BJ, Elhlou S, Pearson NJ, Walker DM, Bayl P, et al. Spatial distribution of lead in human primary teeth as a biomarker of pre- and neonatal lead exposure. Sci Total Environ. 2006;371(1–3):55–62. 10.1016/j.scitotenv.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 226.Shepherd TJ, Dirks W, Manmee C, Hodgson S, Banks DA, Averley P, et al. Reconstructing the life-time lead exposure in children using dentine in deciduous teeth. Sci Total Environ. 2012;425:214–22. 10.1016/j.scitotenv.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 227.Arora M, Austin C, Sarrafpour B, Hernández-Ávila M, Hu H, Wright RO, et al. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One. 2014;9(5):e97805. 10.1371/journal.pone.0097805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hubermont G, Buchet JP, Roels H, Lauwerys R. Placental transfer of lead, mercury and cadmium in women living in a rural area. Int Arch Occ Env Hea. 1978;41(2):117–24. 10.1007/BF00381796. [DOI] [PubMed] [Google Scholar]
- 229.Zhou C, Zhang R, Cai X, Xiao R, Yu H. Trace elements profiles of maternal blood, umbilical cord blood, and placenta in Beijing, China. J Matern Fetal Neonatal Med. 2019;32(11):1755–61. 10.1080/14767058.2017.1416602. [DOI] [PubMed] [Google Scholar]
- 230.Sommar JN, Hedmer M, Lundh T, Nilsson L, Skerfving S, Bergdahl IA. Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J Expo Sci Environ Epidemiol. 2014;24(1):51–7. 10.1038/jes.2013.4. [DOI] [PubMed] [Google Scholar]
- 231.Kostova I, Vassileva C, Dai S, Hower JC, Apostolova D. Influence of surface area properties on mercury capture behaviour of coal fly ashes from some Bulgarian power plants. Int J Coal Geol. 2013;116:227–35. [Google Scholar]
- 232.Gustin MS. Are mercury emissions from geologic sources significant? A status report. Sci Total Environ. 2003;304(1–3):153–67. [DOI] [PubMed] [Google Scholar]
- 233.Martín JAR, Nanos N. Soil as an archive of coal-fired power plant mercury deposition. J Hazard Mater. 2016;308:131–8. [DOI] [PubMed] [Google Scholar]
- 234.Zhang H, Chen J, Zhu L, Yang G, Li D. Anthropogenic mercury enrichment factors and contributions in soils of Guangdong Province, South China. J Geochem Explor. 2014;144:312–9. [Google Scholar]
- 235.Risher J, DeWoskin R. Toxicological Profile for Mercury: Chapter 5. Potential for human exposure. Atlanta, Georgia: U.S. Dept. of health and human services, Public Health Service, Agency for Toxic Substances and Disease Registry; 1999. [Google Scholar]
- 236.Kim K-H, Kabir E, Jahan SA. A review on the distribution of Hg in the environment and its human health impacts. J Hazard Mater. 2016;306:376–85. 10.1016/j.jhazmat.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 237.Bjørklund G, Dadar M, Mutter J, Aaseth J. The toxicology of mercury: current research and emerging trends. Environ Res. 2017;159:545–54. 10.1016/j.envres.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 238.Sakamoto M, Nakamura M, Murata K. Mercury as a global pollutant and mercury exposure assessment and health effects. Nihon Eiseigaku Zasshi. 2018;73(3):258–64. 10.1265/jjh.73.258. [DOI] [PubMed] [Google Scholar]
- 239.Bolan S, Kunhikrishnan A, Seshadri B, Choppala G, Naidu R, Bolan NS, et al. Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ Int. 2017;108:103–18. 10.1016/j.envint.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 240.Raj D, Maiti SK. Sources, toxicity, and remediation of mercury: an essence review. Environ Monit Assess. 2019;191(9):566. 10.1007/s10661-019-7743-2. [DOI] [PubMed] [Google Scholar]
- 241.Halbach K, Mikkelsen Ø, Berg T, Steinnes E. The presence of mercury and other trace metals in surface soils in the Norwegian Arctic. Chemosphere. 2017;188:567–74. [DOI] [PubMed] [Google Scholar]
- 242.Bradley MA, Barst BD, Basu N. A review of mercury bioavailability in humans and fish. Int J Environ Res Pu. 2017;14(2):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tibau AV, Grube BD. Mercury contamination from dental amalgam. J Health Pollut. 2019;9(22):190612. 10.5696/2156-9614-9.22.190612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen X, Xia X, Wu S, Wang F, Guo X. Mercury in urban soils with various types of land use in Beijing. China Environ Pollut. 2010;158(1):48–54. [DOI] [PubMed] [Google Scholar]
- 245.Bjørklund G, Aaseth J, Ajsuvakova OP, Nikonorov AA, Skalny AV, Skalnaya MG, et al. Molecular interaction between mercury and selenium in neurotoxicity. Coord Chem Rev. 2017;332:30–7. 10.1016/j.ccr.2016.10.009. [DOI] [Google Scholar]
- 246.Risher J, DeWoskin R. Toxicological profile for mercury: chapter 2. Health effects. Atlanta, Georgia: U.S. Dept. of health and human services, public health service, agency for toxic substances and disease registry; 1999. [Google Scholar]
- 247.Sunderland EM, Mason RP. Human impacts on open ocean mercury concentrations. Global Biogeochem Cycles. 2007;21(4). [Google Scholar]
- 248.Feng X, Li P, Qiu G, Wang S, Li G, Shang L, et al. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province. China Environ Sci Technol. 2008;42(1):326–32. [DOI] [PubMed] [Google Scholar]
- 249.Zhang H, Feng X, Larssen T, Qiu G, Vogt RD. In inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ Health Persp. 2010;118(9):1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Branco V, Caito S, Farina M, da Rocha JT, Aschner M, Carvalho C. Biomarkers of mercury toxicity: past, present, and future trends. J Toxicol Environ Health B Crit Rev. 2017;20(3):119–54. 10.1080/10937404.2017.1289834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hamann CR, Boonchai W, Wen L, Sakanashi EN, Chu C-Y, Hamann K, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70(2):281–7.e3. 10.1016/j.jaad.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 252.Podgórska A, Puścion-Jakubik A, Grodzka A, Naliwajko SK, Markiewicz-Żukowska R, Socha K. Natural and conventional cosmetics—mercury exposure assessment. Molecules. 2021;26(13):4088. 10.3390/molecules26134088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury— current exposures and clinical manifestations. New Engl J Med. 2003;349(18):1731–7. [DOI] [PubMed] [Google Scholar]
- 254.Decharat S, Phethuayluk P, Maneelok S, Thepaksorn P. Determination of mercury exposure among dental health workers in Nakhon Si Thammarat Province, Thailand. J Toxicol. 2014;2014:401012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.World Health Organization. Mercury. Geneva: World Health Organization; 1976. [Google Scholar]
- 256.Yang L, Zhang Y, Wang F, Luo Z, Guo S, Strähle U. Toxicity of mercury: molecular evidence. Chemosphere. 2020;245:125586. 10.1016/j.chemosphere.2019.125586. [DOI] [PubMed] [Google Scholar]
- 257.Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508. 10.1155/2012/460508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Magos L, Halbach S, Clarkson T. Role of catalase in the oxidation of mercury vapor. Biochem Pharmacol. 1978;27(9):1373–7. [DOI] [PubMed] [Google Scholar]
- 259.Nielsen-Kudsk F. Biological oxidation of elemental mercury. In: Miller MW, Clarkson TW(eds.) Mercury, mercurials and mercaptans. United States: Charles C Thomas,Springfield, IL, USA. [Google Scholar]
- 260.Yaginuma-Sakurai K, Murata K, Iwai-Shimada M, Nakai K, Kurokawa N, Tatsuta N, et al. Hair-to-blood ratio and biological half-life of mercury: experimental study of methylmercury exposure through fish consumption in humans. J Toxicol Sci. 2012;37(1):123–30. [DOI] [PubMed] [Google Scholar]
- 261.Rand MD, Vorojeikina D, van Wijngaarden E, Jackson BP, Scrimale T, Zareba G, et al. Methods for individualized determination of methylmercury elimination rate and de-methylation status in humans following fish consumption. Toxicol Sci. 2016;149(2):385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jo S, Woo HD, Kwon H-J, Oh S-Y, Park J-D, Hong Y-S, et al. Estimation of the biological half-life of methylmercury using a population toxicokinetic model. Int J Environ Res Pu. 2015;12(8):9054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rahola T, Hattula T, Korolainen A, Miettinen J. Elimination of free and protein-bound ionic mercury (203 Hg 2+) in man. Ann Clin Res. 1973;5(4):214–9. [PubMed] [Google Scholar]
- 264.Rooney JP. The retention time of inorganic mercury in the brain—a systematic review of the evidence. Toxicol Appl Pharm. 2014;274(3):425–35. [DOI] [PubMed] [Google Scholar]
- 265.Rebelo FM, Caldas ED. Arsenic, lead, mercury and cadmium: toxicity, levels in breast milk and the risks for breastfed infants. Environ Res. 2016;151:671–88. 10.1016/j.envres.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 266.Cohen JH, Blanchard F, Vischer TL. Class II HLA antigens and rheumatoid factors in rheumatoid polyarthritis. Inverse influence of DR4 and DR7 antigens? Rev Rhum Mal Osteoartic. 1986;53(11):639–41. [PubMed] [Google Scholar]
- 267.Vahter M, Åkesson A, Lind B, Björs U, Schütz A, Berglund M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ Res. 2000;84(2):186–94. [DOI] [PubMed] [Google Scholar]
- 268.Cernichiari E, Brewer R, Myers GJ, Marsh DO, Lapham LW, Cox C, et al. Monitoring methylmercury during pregnancy: maternal hair predicts fetal brain exposure. Neurotoxicology. 1995;16(4):705–10. [PubMed] [Google Scholar]
- 269.Cooke GM. Biomonitoring of human fetal exposure to environmental chemicals in early pregnancy. J Toxicol Environ Health B Crit Rev. 2014;17(4):205–24. [DOI] [PubMed] [Google Scholar]
- 270.LaKind JS, Brent RL, Dourson ML, Kacew S, Koren G, Sonawane B, et al. Human milk biomonitoring data: interpretation and risk assessment issues. J Toxicol Environ Health, A. 2005;68(20):1713–69. [DOI] [PubMed] [Google Scholar]
- 271.Risher J, DeWoskin R. Toxicological profile for mercury. Atlanta, Georgia: U.S. Dept. of health and human services, public health service, agency for toxic substances and disease registry; 1999. [Google Scholar]
- 272.Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50(10):757–64. [DOI] [PubMed] [Google Scholar]
- 273.Sakamoto M, Chan HM, Domingo JL, Oliveira RB, Kawakami S, Murata K. Significance of fingernail and toenail mercury concentrations as biomarkers for prenatal methylmercury exposure in relation to segmental hair mercury concentrations. Environ Res. 2015;136:289–94. 10.1016/j.envres.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 274.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609–62. [DOI] [PubMed] [Google Scholar]
- 275.Rees JR, Sturup S, Chen C, Folt C, Karagas MR. Toenail mercury and dietary fish consumption. J Expo Sci Environ Epidemiol. 2007;17(1):25–30. 10.1038/sj.jes.7500516. [DOI] [PubMed] [Google Scholar]
- 276.Laffont L, Sonke JE, Maurice L, Monrroy SL, Chincheros J, Amouroux D, et al. Hg speciation and stable isotope signatures in human hair as a tracer for dietary and occupational exposure to mercury. Environ Sci Technol. 2011;45(23):9910–6. [DOI] [PubMed] [Google Scholar]
- 277.Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Persp. 2002;110(5):523–6. 10.1289/ehp.02110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B. Mercury exposure and children’s health. Curr Prob Pediatr Ad. 2010;40(8):186–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zalups RK, Barfuss DW, Kostyniak PJ. Altered intrarenal accumulation of mercury in uninephrectomized rats treated with methylmercury chloride. Toxicol Appl Pharm. 1992;115(2):174–82. [DOI] [PubMed] [Google Scholar]
- 280.Caito SW, Jackson BP, Punshon T, Scrimale T, Grier A, Gill SR, et al. Editor’s highlight: variation in methylmercury metabolism and elimination status in humans following fish consumption. Toxicol Sci. 2017;161(2):443–53. 10.1093/toxsci/kfx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Queipo-Abad S, González PR, Martínez-Morillo E, Davis WC, García Alonso JI. Concentration of mercury species in hair, blood and urine of individuals occupationally exposed to gaseous elemental mercury in Asturias (Spain) and its comparison with individuals from a control group formed by close relatives. Sci Total Environ. 2019;672:314–23. 10.1016/j.scitotenv.2019.03.367. [DOI] [PubMed] [Google Scholar]
- 282.Sherman LS, Blum JD, Franzblau A, Basu N. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ Sci Technol. 2013;47(7):3403–9. [DOI] [PubMed] [Google Scholar]
- 283.Berglund M, Lind B, Björnberg KA, Palm B, Einarsson Ö, Vahter M. Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health. 2005;4(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Centers for Disease Control and Prevention. Biomonitoring summary: mercury [updated 4/7/2017]. Available from: https://www.cdc.gov/biomonitoring/Mercury_BiomonitoringSummary.html
- 285.Denkhaus E, Salnikow K. Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hemat. 2002;42(1):35–56. 10.1016/S1040-8428(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 286.Abadin H, Fay M, Wilbur S, Ingerman L, Swarts SG. Toxicological profile for Nickel. Atlanta, Georgia: U.S. Dept. of health and human services, public health service, agency for toxic substances and disease registry; 2005. [Google Scholar]
- 287.Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A. Nickel: Human health and environmental toxicology . Int J Environ Res Pu. 2020;17(3):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zambelli B, Ciurli S. Nickel and Human Health. In: Sigel A, Sigel H, Sigel RK, editors. Metal Ions in Life Sciences: Interrelations between essential metal ions and human diseases. 13. Netherlands: Springer; 2013. [DOI] [PubMed] [Google Scholar]
- 289.Babaahmadifooladi M, Jacxsens L, Van de Wiele T, Du Laing G. Gap analysis of nickel bioaccessibility and bioavailability in different food matrices and its impact on the nickel exposure assessment. Food Res Int. 2020;129:108866. [DOI] [PubMed] [Google Scholar]
- 290.Cempel M, Nikel G. Nickel: a review of its sources and environmental toxicology . Pol J Environ Stud. 2006;15(3). [Google Scholar]
- 291.Abadin H, Fay M, Wilbur SB. Toxicological profile for nickel. Chapter 6: Potential for human exposure. 2005. [Google Scholar]
- 292.Wittsiepe J, Schnell K, Hilbig A, Schrey P, Kersting M, Wilhelm M. Dietary intake of nickel and zinc by young children – results from food duplicate portion measurements in comparison to data calculated from dietary records and available data on levels in food groups. J Trace Elem Med Biol. 2009;23(3):183–94. 10.1016/j.jtemb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 293.Zhao D, Aravindakshan A, Hilpert M, Olmedo P, Rule AM, Navas-Acien A, et al. Metal/metalloid levels in electronic cigarette liquids, aerosols, and human biosamples: a systematic review. Environ Health Persp. 2020;128(3):036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhao D, Navas-Acien A, Ilievski V, Slavkovich V, Olmedo P, Adria-Mora B, et al. Metal concentrations in electronic cigarette aerosol: effect of open-system and closed-system devices and power settings. Environ Res. 2019;174:125–34. 10.1016/j.envres.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Oskarsson A, Tjälve H. The distribution and metabolism of nickel carbonyl in mice. Occup Environ Med. 1979;36(4):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Donald GB, Donald B. Nickel. Clin Toxicol. 1999;37:239–58. [DOI] [PubMed] [Google Scholar]
- 297.Costa M, Abbracchio MP, Simmons-Hansen J. Factors influencing the phagocytosis, neoplastic transformation, and cytotoxicity of particulate nickel compounds in tissue culture systems. Toxicol Appl Pharm. 1981;60(2):313–23. [DOI] [PubMed] [Google Scholar]
- 298.Tallkvist J, Bowlus CL, Lönnerdal B. Effect of iron treatment on nickel absorption and gene expression of the divalent metal transporter (DMT1) by human intestinal Caco-2 cells. Pharmacol Toxicol. 2003;92(3):121–4. [DOI] [PubMed] [Google Scholar]
- 299.Moody RP, Joncas J, Richardson M, Petrovic S, Chu I. Contaminated soils (II): in vitro dermal absorption of nickel (Ni-63) and mercury (Hg-203) in human skin. J Toxicol Environ Health A. 2009;72(8):551–9. 10.1080/15287390802706322. [DOI] [PubMed] [Google Scholar]
- 300.Crosera M, Adami G, Mauro M, Bovenzi M, Baracchini E, Larese FF. In vitro dermal penetration of nickel nanoparticles. Chemosphere. 2016;145:301–6. 10.1016/j.chemosphere.2015.11.076. [DOI] [PubMed] [Google Scholar]
- 301.World Health Organization. Regional office for Europe. Air quality guidelines for Europe. 2nd ed: World Health Organization. Regional Office for Europe. 2000. [Google Scholar]
- 302.Tjälve H, Jasim S, Oskarsson A. Nickel mobilization by sodium diethyldithiocarbamate in nickel-carbonyl-treated mice. IARC Sci Publ. 1984;53:311–20. [PubMed] [Google Scholar]
- 303.Kasprzak KS, Waalkes MP, Poirier LA. Effects of magnesium acetate on the toxicity of nickelous acetate in rats. Toxicology. 1986;42(1):57–68. [DOI] [PubMed] [Google Scholar]
- 304.Owumi SE, Olayiwola YO, Alao GE, Gbadegesin MA, Odunola OA. Cadmium and nickel co-exposure exacerbates genotoxicity and not oxido-inflammatory stress in liver and kidney of rats: Protective role of omega-3 fatty acid. Environ Toxicol. 2020;35(2):231–41. 10.1002/tox.22860. [DOI] [PubMed] [Google Scholar]
- 305.Johansson A, Curstedt T, Jarstrand C, Robertson B, Camner P. Effects on the rabbit lung of combined exposure to nickel and trivalent chromium. J Aerosol Sci. 1988;19(7):1075–8. [Google Scholar]
- 306.Arenas IA, Navas-Acien A, Ergui I, Lamas GA. Enhanced vasculotoxic metal excretion in post-myocardial infarction patients following a single edetate disodium-based infusion. Environ Res. 2017;158:443–9. 10.1016/j.envres.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 307.Abadin H, Fay M, Wilbur SB. Toxicological profile for Nickel. Chapter 3: health effects. Atlanta, Georgia: U.S. dept. of health and human services, public health service, agency for toxic substances and disease registry; 2005. [Google Scholar]
- 308.Sunderman JRFW, Aitio A, Morgan LG, Norseth T. Biological monitoring of nickel. Toxicol Ind Health. 1986;2(1):17–78. [DOI] [PubMed] [Google Scholar]
- 309.Sunderman FW. Biological monitoring of nickel in humans. Scand J Work Environ Health. 1993;19:34–8. [PubMed] [Google Scholar]
- 310.Morgan L, Rouge P. Biological monitoring in nickel refinery workers. IARC Sci Publ. 1984;53:507–20. [PubMed] [Google Scholar]
- 311.Bernacki E, Zygowicz E, Sunderman F. Fluctuations of nickel concentrations in urine of electroplating workers. Ann Clin Lab Sci. 1980;10(1):33–9. [PubMed] [Google Scholar]
- 312.Templeton DM, Sunderman FW, Herber RFM. Tentative reference values for nickel concentrations in human serum, plasma, blood, and urine: evaluation according to the TRACY protocol. Sci Total Environ. 1994;148(2):243–51. 10.1016/0048-9697(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 313.Reichrtová E, Dorociak F, Palkovicová L. Sites of lead and nickel accumulation in the placental tissue. Hum Exp Toxicol. 1998;17(3):176–81. 10.1177/096032719801700309. [DOI] [PubMed] [Google Scholar]
- 314.Harper C, Llados F. Toxicological profile for tin and tin compounds. 2005. [PubMed]
- 315.Okoro HK, Fatoki OS, Adekola FA, Ximba BJ, Snyman RG, Opeolu B. Human exposure, biomarkers, and fate of organotins in the environment. Rev Environ Contam Toxicol. 2011;213:27–54. 10.1007/978-1-4419-9860-6_2. [DOI] [PubMed] [Google Scholar]
- 316.Cao DJ, Aldy K, Hsu S, McGetrick M, Verbeck G, De Silva I, et al. Review of health consequences of electronic cigarettes and the outbreak of electronic cigarette, or vaping, product use-associated lung injury. J Med Toxicol. 2020;16(3):295–310. 10.1007/s13181-020-00772-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.National Institute of Diabetes Digestive Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 318.Sadiki A-D, Williams DT. A study on organotin levels in Canadian drinking water distributed through PVC pipesa. Chemosphere. 1999;38(7):1541–8. [DOI] [PubMed] [Google Scholar]
- 319.Izah SC, Inyang IR, Angaye TCN, Okowa IP. A review of heavy metal concentration and potential health implications of beverages consumed in Nigeria. Toxics. 2016;5(1). 10.3390/toxics5010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Azenha M, Vasconcelos MT. Butyltin compounds in Portuguese wines. J Agr Food Chem. 2002;50(9):2713–6. [DOI] [PubMed] [Google Scholar]
- 321.Deshwal GK, Panjagari NR. Review on metal packaging: materials, forms, food applications, safety and recyclability. J Food Sci Technol. 2020;57(7):2377–92. 10.1007/s13197-019-04172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Chien L-C, Hung T-C, Choang K-Y, Yeh C-Y, Meng P-J, Shieh M-J, et al. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci Total Environ. 2002;285(1–3):177–85. [DOI] [PubMed] [Google Scholar]
- 323.Rantakokko P, Turunen A, Verkasalo PK, Kiviranta H, Männistö S, Vartiainen T. Blood levels of organotin compounds and their relation to fish consumption in Finland. Sci Total Environ. 2008;399(1–3):90–5. [DOI] [PubMed] [Google Scholar]
- 324.Lehmler HJ, Gadogbe M, Liu B, Bao W. Environmental tin exposure in a nationally representative sample of U.S. adults and children: The National Health and Nutrition Examination Survey 2011–2014. Environ Pollut. 2018;240:599–606. 10.1016/j.envpol.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Johnson MA, Greger J. Effects of dietary tin on tin and calcium metabolism of adult males. Am J Clin Nutr. 1982;35(4):655–60. [DOI] [PubMed] [Google Scholar]
- 326.Gadogbe M, Bao W, Wels BR, Dai SY, Santillan DA, Santillan MK, et al. Levels of tin and organotin compounds in human urine samples from Iowa, United States. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2019;54(9):884–90. 10.1080/10934529.2019.1605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Domingo JL. Reproductive and developmental toxicity of natural and depleted uranium: a review. Reprod Toxicol. 2001;15(6):603–9. [DOI] [PubMed] [Google Scholar]
- 328.Faroon O, Ingerman L, Roney N, Scinicariello F, Wilbur SB. Toxicological profile for uranium. chapter 3: health effects. Atlanta, GA: U.S. dept. of health and human services, public health service, agency for toxic substances and disease registry; 2013. [PubMed] [Google Scholar]
- 329.Liesch T, Hinrichsen S, Goldscheider N. Uranium in groundwater–fertilizers versus geogenic sources. Sci Total Environ. 2015;536:981–95. 10.1016/j.scitotenv.2015.05.133. [DOI] [PubMed] [Google Scholar]
- 330.Hegedűs M, Tóth-Bodrogi E, Németh S, Somlai J, Kovács T. Radiological investigation of phosphate fertilizers: leaching studies. J Environ Radioact. 2017;173:34–43. [DOI] [PubMed] [Google Scholar]
- 331.Wang S, Ran Y, Lu B, Li J, Kuang H, Gong L, et al. A Review of uranium-induced reproductive toxicity. Biol Trace Elem Res. 2020;196(1):204–13. 10.1007/s12011-019-01920-2. [DOI] [PubMed] [Google Scholar]
- 332.Souidi M, Tissandie E, Racine R, Soussan HB, Rouas C, Grignard E, et al. Uranium: propriétés et effets biologiques après contamination interne. Annales de Biologie Clinique. 2009;67(1):23–38. [DOI] [PubMed] [Google Scholar]
- 333.Banning A, Demmel T, Rüde TR, Wrobel M. Groundwater uranium origin and fate control in a river valley aquifer. Environ Sci Technol. 2013;47(24):13941–8. [DOI] [PubMed] [Google Scholar]
- 334.Bjørklund G, Christophersen OA, Chirumbolo S, Selinus O, Aaseth J. Recent aspects of uranium toxicology in medical geology. Environ Res. 2017;156:526–33. 10.1016/j.envres.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 335.Bjørklund G, Semenova Y, Pivina L, Dadar M, Rahman MM, Aaseth J, et al. Uranium in drinking water: a public health threat. Arch Toxicol. 2020;94(5):1551–60. [DOI] [PubMed] [Google Scholar]
- 336.Ma M, Wang R, Xu L, Xu M, Liu S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: lessons from the past two decades. Environ Int. 2020;145:106107. 10.1016/j.envint.2020.106107. [DOI] [PubMed] [Google Scholar]
- 337.Redvers N, Chischilly AM, Warne D, Pino M, Lyon-Colbert A. Uranium exposure in American Indian communities: health, policy, and the way forward. Environ Health Persp. 2021;129(3):035002. 10.1289/EHP7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sobel M, Sanchez TR, Zacher T, Mailloux B, Powers M, Yracheta J, et al. Spatial relationship between well water arsenic and uranium in Northern Plains native lands. Environ Pollut. 2021;287:117655. 10.1016/j.envpol.2021.117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Credo J, Torkelson J, Rock T, Ingram JC. Quantification of elemental contaminants in unregulated water across western Navajo Nation. Int J Environ Res Public Health. 2019;16(15). 10.3390/ijerph16152727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lai JL, Liu ZW, Li C, Luo XG. Analysis of accumulation and phytotoxicity mechanism of uranium and cadmium in two sweet potato cultivars. J Hazard Mater. 2021;409:124997. 10.1016/j.jhazmat.2020.124997. [DOI] [PubMed] [Google Scholar]
- 341.Bellés M, Linares V, Perelló G, Domingo JL. Human dietary exposure to uranium in Catalonia. Spain Biol Trace Elem Res. 2013;152(1):1–8. [DOI] [PubMed] [Google Scholar]
- 342.Keith LS, Faroon OM, Fowler BA. Chapter 59 - Uranium. In: Nordberg GF, Fowler BA, Nordberg M, editors. Handbook on the toxicology of metals. 4th ed. San Diego: Academic Press; 2015. p. 1307–45. [Google Scholar]
- 343.Yue Y-C, Li M-H, Wang H-B, Zhang B-L, He W The toxicological mechanisms and detoxification of depleted uranium exposure. Environ Health Prev Med. 2018;23(1):18. 10.1186/s12199-018-0706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Petitot F, Frelon S, Moreels AM, Claraz M, Delissen O, Tourlonias E, et al. Incorporation and distribution of uranium in rats after a contamination on intact or wounded skin. Health Phys. 2007;92(5):464–74. [DOI] [PubMed] [Google Scholar]
- 345.Guéguen Y, Roy L, Hornhardt S, Badie C, Hall J, Baatout S, et al. Biomarkers for uranium risk assessment for the development of the CURE (Concerted Uranium Research in Europe) molecular epidemiological protocol. Radiat Res. 2017;187(1):107–27. 10.1667/RR14505.1. [DOI] [PubMed] [Google Scholar]
- 346.Stradling GN, Smith H, Cooper JR, Ham SE, Cooke N, Sedgwick D, et al. Factors affecting the abundance of uranium isotopes in body tissues and excreta following the deposition of enriched uranium dioxide in the lungs--the radiological implications. Health Physics. 1984;46(2):434–8. [PubMed] [Google Scholar]
- 347.Konietzka R. Gastrointestinal absorption of uranium compounds – a review. Regul Toxicol Pharmacol. 2015;71(1):125–33. 10.1016/j.yrtph.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 348.International Commission on Radiological Protection. Age-dependent doses to members of the public from intake of radionuclides: Part 4 Inhalation dose coefficients. Ann ICRP. 1995;25(3–4):i–i. 10.1016/s0146-6453(00)80008-1. [DOI] [PubMed] [Google Scholar]
- 349.Leggett R, Harrison J. Fractional absorption of ingested uranium in humans. Health Phys. 1995;68(4):484–98. [DOI] [PubMed] [Google Scholar]
- 350.Spencer H, Osis D, Fisenne IM, Perry PM, Harley NH. Measured intake and excretion patterns of naturally occurring, and calcium in humans. Radiat Res. 1990;124(1):90–5. [PubMed] [Google Scholar]
- 351.Wrenn ME, Singh NP, Ruth H, Rallison ML, Burleigh DP. Gastrointestinal absorption of soluble uranium from drinking water by man. Radiat Prot Dosimet. 1989;26(1–4):119–22. 10.1093/oxfordjournals.rpd.a080391. [DOI] [Google Scholar]
- 352.Zhu G, Tan M, Li Y, Xiang X, Hu H, Zhao S. Accumulation and distribution of uranium in rats after implantation with depleted uranium fragments. J Radiat Res. 2009;50(3):183–92. [DOI] [PubMed] [Google Scholar]
- 353.Busby C. Uranium and health: the health effects of exposure to uranium and uranium weapons fallout. Recommendations of the European Committee on Radiation Risk (ECRR), Brussels. 2010. [Google Scholar]
- 354.Cooper JR, Stradling GN, Smith H, Ham SE. The behaviour of uranium-233 oxide and uranyl-233 nitrate in rats. Int J Radiat Biol Re. 1982;41(4):421–33. [DOI] [PubMed] [Google Scholar]
- 355.Dounce ALFJ. The chemistry of uranium compounds. In: Voegtlin C, Hodge HC, editors. Pharmacology and toxicology of uranium compounds. 55. New York, NY: McGraw-Hill Book Co., Inc.; 1949. pp, 83–4. [Google Scholar]
- 356.Stevens W, Bruenger F, Atherton D, Smith J, Taylor G. The distribution and retention of hexavalent 233U in the beagle. Radiat Res. 1980;83(1):109–26. [PubMed] [Google Scholar]
- 357.Wedeen R. Renal diseases of occupational origin. Occup Med. 1992;7(3):449–63. [PubMed] [Google Scholar]
- 358.Vicente-Vicente L, Quiros Y, Perez-Barriocanal F, Lopez-Novoa JM, Lopez-Hernandez FJ, Morales AI. Nephrotoxicity of uranium: pathophysiological, diagnostic and therapeutic perspectives. Toxicol Sci: Off J Soc Toxicol. 2010;118(2):324–47. 10.1093/toxsci/kfq178. [DOI] [PubMed] [Google Scholar]
- 359.Homma-Takeda S, Kokubo T, Terada Y, Suzuki K, Ueno S, Hayao T, et al. Uranium dynamics and developmental sensitivity in rat kidney. J Appl Toxicol. 2013;33(7):685–94. [DOI] [PubMed] [Google Scholar]
- 360.McDiarmid MA, Hooper FJ, Squibb K, McPhaul K. The utility of spot collection for urinary uranium determinations in depleted uranium exposed Gulf War veterans. Health Phys. 1999;77(3):261–4. [DOI] [PubMed] [Google Scholar]
- 361.Karpas Z, Lorber A, Sela H, Paz-Tal O, Hagag Y, Kurttio P, et al. Measurement of the 234U/238U ratio by MC-ICPMS in drinking water, hair, nails, and urine as an indicator of uranium exposure source. Health Phys. 2005;89(4):315–21. [DOI] [PubMed] [Google Scholar]
- 362.Karpas Z. Uranium bioassay–beyond urinalysis. Health Phys. 2001;81(4):460–3. [DOI] [PubMed] [Google Scholar]
- 363.Karpas Z, Paz-Tal O, Lorber A, Salonen L, Komulainen H, Auvinen A, et al. Urine, hair, and nails as indicators for ingestion of uranium in drinking water. Health Phys. 2005;88(3):229–42. [DOI] [PubMed] [Google Scholar]
- 364.Muikku M, Puhakainen M, Heikkinen T, Ilus T. The mean concentration of uranium in drinking water, urine, and hair of the occupationally unexposed Finnish working population. Health Phys. 2009;96(6):646–54. [DOI] [PubMed] [Google Scholar]
- 365.Ballou J, Gies R, Case A, Haggard D, Buschbom R, Ryan J. Deposition and early disposition of inhaled uranium-233 uranyl nitrate and uranium-232 uranyl nitrate in the rat. Health Phys. 1986;51:755–72. [DOI] [PubMed] [Google Scholar]
- 366.Downs WL, Wilson HB, Sylvester GE, Leach LJ, Maynard EA. Excretion of uranium by rats following inhalation of uranium dioxide. Health Phys. 1967;13(5):445–53. [DOI] [PubMed] [Google Scholar]
- 367.Leach LJ, Gelein RM, Panner BJ, Yulie CL, Cox CC, Balys MM, et al. Acute toxicity of the hydrolysis products of uranium hexafluoride (UF/sub6/) when inhaled by the rat and guinea pig. Final report. United States; 1984. Contract No.: K/SUB-81– 9039/3; ON: DE84011539. [Google Scholar]
- 368.Morrow P, Gelein R, Beiter H, Scott J, Picano J, Yuile C. Inhalation and intravenous studies of UF6/UO2F2 in dogs. Health Phys. 1982;43(6):859–73. [DOI] [PubMed] [Google Scholar]
- 369.West C, Scott L. Uranium cases showing long chest burden retention-an updating. Health Phys. 1969;17(6):781–91. [DOI] [PubMed] [Google Scholar]
- 370.Wrenn M, Durbin PW, Howard B, Lipsztein J, Rundo J, Still ET, et al. Metabolism of ingested U and Ra. Health Phys. 1985;48(5):601–33. [DOI] [PubMed] [Google Scholar]
- 371.Stradling G, Stather J, Gray S, Moody J, Ellender M, Hodgson A, et al. Metabolism of uranium in the rat after inhalation of two industrial forms of ore concentrate: the implications for occupational exposure. Hum Toxicol. 1987;6(5):385–93. [DOI] [PubMed] [Google Scholar]
- 372.Marco R, Katorza E, Gonen R, German U, Tshuva A, Pelled O, et al. Normalisation of spot urine samples to 24-h collection for assessment of exposure to uranium. Radiat Prot Dosimet. 2008;130(2):213–23. [DOI] [PubMed] [Google Scholar]
- 373.May LM, Heller J, Kalinsky V, Ejnik J, Cordero S, Oberbroekling KJ, et al. Military deployment human exposure assessment: urine total and isotopic uranium sampling results. J Toxicol Environ Health A. 2004;67(8–10):697–714. 10.1080/15287390490428189. [DOI] [PubMed] [Google Scholar]
- 374.Dang HS, Pullat VR, Pillai KC. Determining the normal concentration of uranium in urine and application of the data to its biokinetics. Health Phys. 1992;62(6):562–6. 10.1097/00004032-199206000-00010. [DOI] [PubMed] [Google Scholar]
- 375.Orloff KG, Mistry K, Charp P, Metcalf S, Marino R, Shelly T, et al. Human exposure to uranium in groundwater. Environ Res. 2004;94(3):319–26. 10.1016/S0013-9351(03)00115-4. [DOI] [PubMed] [Google Scholar]
- 376.Salcedo-Bellido I, Gutiérrez-González E, García-Esquinas E, de Larrea-Baz NF, Navas-Acien A, Téllez-Plaza M, et al. Toxic metals in toenails as biomarkers of exposure: a review. Environ Res. 2021;197:111028. 10.1016/j.envres.2021.111028. [DOI] [PubMed] [Google Scholar]
- 377.Kurttio P, Komulainen H, Leino A, Salonen L, Auvinen A, Saha H. Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environ Health Persp. 2005;113(1):68–72. 10.1289/ehp.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Williams M, Todd GD, Roney N, Crawford J, Coles C, McClure PR, et al. Agency for toxic substances and disease registry (ATSDR) Toxicological profiles. Toxicological profile for Manganese. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2012. [PubMed] [Google Scholar]
- 379.Institute of Medicine. Dietary reference intakes for vitamin a, vitamin k, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: The National Academies Press; 2001. p. 800. [PubMed] [Google Scholar]
- 380.O’Neal SL, Zheng W. Manganese toxicity upon overexposure: a decade in review. Current environmental health reports. 2015;2(3):315–28. 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–28. 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- 382.Post JE. Manganese oxide minerals: crystal structures and economic and environmental significance. P Natl Acad Sci. 1999;96(7):3447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Williams M, Todd GD, Roney N, Crawford J, Coles C, McClure PR, et al. Agency for toxic substances and disease registry (ATSDR) toxicological profiles. Toxicological profile for Manganese: Chapter 6: potential for human exposure. Atlanta (GA): agency for toxic substances and disease registry (US); 2012. [PubMed] [Google Scholar]
- 384.Gale EM, Wey H-Y, Ramsay I, Yen Y-F, Sosnovik DE, Caravan P. A Manganese-based alternative to gadolinium: contrastenhanced MR angiography, excretion, pharmacokinetics, and metabolism. Radiology. 2018;286(3):865–72. 10.1148/radiol.2017170977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhou IY, Ramsay IA, Ay I, Pantazopoulos P, Rotile NJ, Wong A, et al. Positron emission tomography-magnetic resonance imaging pharmacokinetics, in vivo biodistribution, and whole-body elimination of Mn-PyC3A. Invest Radiol. 2021;56(4):261–70. 10.1097/rli.0000000000000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26(4–5):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.World Health Organization. Manganese in drinking water: background document for development of WHO guidelines for drinking-water quality. World Health Organization; 2021. [Google Scholar]
- 388.Frisbie SH, Ortega R, Maynard DM, Sarkar B. The concentrations of arsenic and other toxic elements in Bangladesh’s drinking water. Environ Health Persp. 2002;110(11):1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Bowler RM, Gocheva V, Harris M, Ngo L, Abdelouahab N, Wilkinson J, et al. Prospective study on neurotoxic effects in manganese-exposed bridge construction welders. Neurotoxicology. 2011;32(5):596–605. [DOI] [PubMed] [Google Scholar]
- 390.Huang C-C, Chu N-S, Lu C-S, Wang J-D, Tsai J-L, Tzeng J-L, et al. Chronic manganese intoxication. Arch Neurol. 1989;46(10):1104–6. [DOI] [PubMed] [Google Scholar]
- 391.Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front Biosci (Landmark Ed). 2018;23:1655–79. 10.2741/4665. [DOI] [PubMed] [Google Scholar]
- 392.Leblondel G, Allain P. Manganese transport by Caco-2 cells. Biol Trace Elem Res. 1999;67(1):13–28. 10.1007/bf02784271. [DOI] [PubMed] [Google Scholar]
- 393.Mena I, Horiuchi K, Burke K, Cotzias GC. Chronic manganese poisoning. Individual susceptibility and absorption of iron. Neurology. 1969;19(10):1000–6. 10.1212/wnl.19.10.1000. [DOI] [PubMed] [Google Scholar]
- 394.Ye Q, Park JE, Gugnani K, Betharia S, Pino-Figueroa A, Kim J. Influence of iron metabolism on manganese transport and toxicity. Metallomics. 2017;9(8):1028–46. 10.1039/c7mt00079k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Finley JW, Johnson PE, Johnson L. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am J Clin Nutr. 1994;60(6):949–55. [DOI] [PubMed] [Google Scholar]
- 396.Raghib MH, Wai-Yee C, Rennert MO. Comparative biological availability of manganese from extrinsically labelled milk diets using sucking rats as a model. Brit J Nutr. 1986;55(1):49–58. [DOI] [PubMed] [Google Scholar]
- 397.Davidsson L, Cederblad A, Lönnerdal B, Sandström B. The effect of individual dietary components on manganese absorption in humans. Am J Clin Nutr. 1991;54(6):1065–70. [DOI] [PubMed] [Google Scholar]
- 398.Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, et al. Manganese homeostasis in the nervous system. J Neurochem. 2015;134(4):601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Leavens TL, Rao D, Andersen ME, Dorman DC. Evaluating transport of manganese from olfactory mucosa to striatum by pharmacokinetic modeling. Toxicol Sci. 2007;97(2):265–78. [DOI] [PubMed] [Google Scholar]
- 400.Lucchini R, Dorman D, Elder A, Veronesi B. Neurological impacts from inhalation of pollutants and the nose–brain connection. Neurotoxicology. 2012;33(4):838–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Krebs N, Langkammer C, Goessler W, Ropele S, Fazekas F, Yen K, et al. Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J Trace Elem Med Biol. 2014;28(1):1–7. [DOI] [PubMed] [Google Scholar]
- 402.Rahil-Khazen R, Bolann BJ, Myking A, Ulvik RJ. Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES). J Trace Elem Med Biol. 2002;16(1):15–25. [DOI] [PubMed] [Google Scholar]
- 403.Liu Y, Byrne P, Wang H, Koltick D, Zheng W, Nie LH. A compact DD neutron generator–based NAA system to quantify manganese (Mn) in bone in vivo. Physiol Meas. 2014;35(9):1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Reaney SH, Kwik-Uribe CL, Smith DR. Manganese oxidation state and its implications for toxicity. Chem Res Toxicol. 2002;15(9):1119–26. [DOI] [PubMed] [Google Scholar]
- 405.Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, et al. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology. 2007;28(1):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Omokhodion FO, Howard JM. Trace elements in the sweat of acclimatized persons. Clin Chim Acta. 1994;231(1):23–8. [DOI] [PubMed] [Google Scholar]
- 407.Davis CD, Zech L, Greger J. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. P Soc Exp Biol Med. 1993;202(1):103–8. [DOI] [PubMed] [Google Scholar]
- 408.Coles C, Crawford J, McClure PR, Roney N, Todd GD. Toxicological profile for manganese. Chapter 3: Health effects. 2012. [PubMed] [Google Scholar]
- 409.Bertinchamps A, Miller S, Cotzias G. Interdependence of routes excreting manganese. Am J Physiol. 1966;211(1):217–24. [DOI] [PubMed] [Google Scholar]
- 410.Malecki EA, Radzanowski GM, Radzanowski TJ, Gallaher DD, Greger J. Biliary manganese excretion in conscious rats is affected by acute and chronic manganese intake but not by dietary fat. J Nutr. 1996;126(2):489–98. [DOI] [PubMed] [Google Scholar]
- 411.Stastny D, Vogel RS, Picciano M. Manganese intake and serum manganese concentration of human milk-fed and formula-fed infants. Am J Clin Nutr. 1984;39(6):872–8. [DOI] [PubMed] [Google Scholar]
- 412.Grünecker B, Kaltwasser S, Zappe A, Bedenk B, Bicker Y, Spoormaker V, et al. Regional specificity of manganese accumulation and clearance in the mouse brain: implications for manganese-enhanced MRI. NMR Biomed. 2013;26(5):542–56. [DOI] [PubMed] [Google Scholar]
- 413.O’Neal SL, Zheng W. Manganese toxicity upon overexposure: a decade in review. Current environmental health reports. 2015;2(3):315–28. 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695(1):53–8. [DOI] [PubMed] [Google Scholar]
- 415.O’Neal SL, Hong L, Fu S, Jiang W, Jones A, Nie LH, et al. Manganese accumulation in bone following chronic exposure in rats: steady-state concentration and half-life in bone. Toxicol Lett. 2014;229(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Yin S, Wang C, Wei J, Wang D, Jin L, Liu J, et al. Essential trace elements in placental tissue and risk for fetal neural tube defects. Environ Int. 2020;139:105688. 10.1016/j.envint.2020.105688. [DOI] [PubMed] [Google Scholar]
- 417.Maccani JZ, Koestler DC, Houseman EA, Armstrong DA, Marsit CJ, Kelsey KT. DNA methylation changes in the placenta are associated with fetal manganese exposure. Reprod Toxicol. 2015;57:43–9. 10.1016/j.reprotox.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Baldwin M, Mergler D, Larribe F, Bélanger S, Tardif R, Bilodeau L, et al. Bioindicator and exposure data for a population based study of manganese. Neurotoxicology. 1999;20(2–3):343–53. [PubMed] [Google Scholar]
- 419.Järvisalo J, Olkinuoral M, Kiilunen M, Kivistö H, Ristola P, Tossavainen A, et al. Urinary and blood manganese in occupationally nonexposed populations and in manual metal are welders of mild steel. Int Arch Occ Env Hea. 1992;63(7):495–501. [DOI] [PubMed] [Google Scholar]
- 420.Roels H, Ghyselen P, Buchet J-P, Ceulemans E, Lauwerys R. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Occup Environ Med. 1992;49(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, et al. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50(11):801–11. 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- 422.Roels H, Lauwerys R, Genet P, Sarhan MJ, de Fays M, Hanotiau I, et al. Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am J Ind Med. 1987;11(3):297–305. [DOI] [PubMed] [Google Scholar]
- 423.Smyth LT, Ruhf R, Whitman N, Dugan T. Clinical manganism and exposure to manganese in the production and processing of ferromanganese alloy. J Occup Environ Med. 1973;15(2):101–9. [PubMed] [Google Scholar]
- 424.Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats . Toxicol Sci. 2000;54(2):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Myers JE, Thompson ML, Naik I, Theodorou P, Esswein E, Tassell H, et al. The utility of biological monitoring for manganese in ferroalloy smelter workers in South Africa. Neurotoxicology. 2003;24(6):875–83. [DOI] [PubMed] [Google Scholar]
- 426.Apostoli P, Lucchini R, Alessio L. Are current biomarkers suitable for the assessment of manganese exposure in individual workers? Am J Ind Med. 2000;37(3):283–90. [DOI] [PubMed] [Google Scholar]
- 427.Bader M, Dietz M, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int Arch Occ Env Hea. 1999;72(8):521–7. [DOI] [PubMed] [Google Scholar]
- 428.Liu W, Xin Y, Li Q, Shang Y, Ping Z, Min J, et al. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: a systematic review and metaanalysis. Environ Health. 2020;19(1):104. 10.1186/s12940-020-00659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Lydén A, Larsson BS, Lindquist NG. Melanin affinity of manganese. Acta Pharmacol Tox. 1984;55(2):133–8. [DOI] [PubMed] [Google Scholar]
- 430.Hurley LS, Keen CL. 6 - Manganese. In: Mertz W, editor. Trace elements in human and animal nutrition. 5th ed. San Diego: Academic Press; 1987. p. 185–223. [Google Scholar]
- 431.Sturaro A, Parvoli G, Doretti L, Allegri G, Costa C. The influence of color, age, and sex on the content of zinc, copper, nickel, manganese, and lead in human hair. Biol Trace Elem Res. 1994;40(1):1–8. [DOI] [PubMed] [Google Scholar]
- 432.Austin C, Richardson C, Smith D, Arora M. Tooth manganese as a biomarker of exposure and body burden in rats. Environ Res. 2017;155:373–9. 10.1016/j.envres.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Signes-Pastor AJ, Bouchard MF, Baker E, Jackson BP, Karagas MR. Toenail manganese as biomarker of drinking water exposure: a reliability study from a US pregnancy cohort. J Expo Sci Environ Epidemiol. 2019;29(5):648–54. 10.1038/s41370-018-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Zheng W, Fu SX, Dydak U, Cowan DM. Biomarkers of manganese intoxication. Neurotoxicology. 2011;32(1):1–8. 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Todd GD, Keith SMS, Faroon O, Melanie Buser MPH, Ingerman L, Hard C, et al. Toxicological profile for molybdenum. Chapter 5: potential for human exposure. Atlanta, GA: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2020. [Google Scholar]
- 436.Barceloux DG, Barceloux D. Molybdenum. J Toxicol-Clin Toxic. 1999;37(2):231–7. 10.1081/CLT-100102422. [DOI] [PubMed] [Google Scholar]
- 437.Fitzgerald D, Nicholson R, Regoli L. Environmental management criteria for molybdenum and selenium: a review relevant to the mining industry. British Columbia Mine Reclamation Symposium; University of British Columbia: Norman B. Keevil Institute of Mining Engineering; 2008. [Google Scholar]
- 438.Goldberg S, Lesch SM, Suarez DL. Predicting molybdenum adsorption by soils using soil chemical parameters in the constant capacitance model. Soil Sci Soc Am J. 2002;66(6):1836–42. [Google Scholar]
- 439.Polyak DE. Molybdenum. Minerals yearbook. I. Reston, VA: U.S. Department of the Interior, U.S. Geological Survey; 2018. [Google Scholar]
- 440.Kaiser BN, Gridley KL, Ngaire Brady J, Phillips T, Tyerman SD. The role of molybdenum in agricultural plant production. Ann Bot. 2005;96(5):745–54. 10.1093/aob/mci226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Todd GD, Keith SMS, Faroon O, Melanie Buser MPH, Ingerman L, Hard C, et al. Toxicological profile for molybdenum. Atlanta, GA: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2020. [Google Scholar]
- 442.National Academies of Sciences Engineering and Medicine. Molybdenum-99 for medical imaging. Washington, DC: The National Academies Press; 2016. p. 263. [PubMed] [Google Scholar]
- 443.Rajagopalan K. Molybdenum: an essential trace element in human nutrition. Annu Rev Nutr. 1988;8(1):401–27. [DOI] [PubMed] [Google Scholar]
- 444.BGS and DPHE. Arsenic contamination of groundwater in Bangladesh. British Geological Survey, Keyworth, 2001. British Geological Survey Report WC/00/19. [Google Scholar]
- 445.Turnlund JR, Keyes WR, Peiffer GL. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men at five intakes of dietary molybdenum. Am J Clin Nutr. 1995;62(4):790–6. 10.1093/ajcn/62.4.790. [DOI] [PubMed] [Google Scholar]
- 446.Giussani A, Arogunjo AM, Cantone MC, Tavola F, Veronese I. Rates of intestinal absorption of molybdenum in humans. App Radiat Isotopes. 2006;64(6):639–44. [DOI] [PubMed] [Google Scholar]
- 447.Werner E, Giussani A, Heinrichs U, Roth P, Greim H. Biokinetic studies in humans with stable isotopes as tracers. Part 2: Uptake of molybdenum from aqueous solutions and labelled foodstuffs. Isotopes Environ Health Stud. 1998;34(3):297–301. 10.1080/10256019808234063. [DOI] [PubMed] [Google Scholar]
- 448.Novotny JA, Turnlund JR. Molybdenum intake influences molybdenum kinetics in men. J Nutr. 2007;137(1):37–42. [DOI] [PubMed] [Google Scholar]
- 449.Tipton IH, Cook MJ. Trace elements in human tissue Part II. Adult subjects from the United States. Health Phys. 1963;9(2):103–45. [DOI] [PubMed] [Google Scholar]
- 450.Tipton I, Schroeder H, Perry H Jr, Cook M. Trace elements in human tissue Part III. Subjects from Africa, the Near and Far East and Europe. Health Phys. 1965;11(5):403–51. [DOI] [PubMed] [Google Scholar]
- 451.Sorensen LB, Archambault M. Visualization of liver by scanning with mo99 (molybdate) as tracer. J Lab Clin Med 1963;62(2):330. [PubMed] [Google Scholar]
- 452.Sumino K, Hayakawa K, Shibata T, Kitamura S. Heavy metals in normal Japanese tissues: amounts of 15 heavy metals in 30 subjects. Arch Environ Health. 1975;30(10):487–94. [DOI] [PubMed] [Google Scholar]
- 453.Yoo YC, Lee SK, Yang JY, In SW, Kim KW, Chung KH, et al. Organ distribution of heavy metals in autopsy material from normal Korean. J Health Sci. 2002;48(2):186–94. [Google Scholar]
- 454.Zeisler R, Greenberg R, Stone S. Radiochemical and instrumental neutron activation analysis procedures for the determination of low level trace elements in human livers. J Radioanal Nucl Ch. 1988;124(1):47–63. [Google Scholar]
- 455.Iyengar GV, Kollmer WE, Bowen HJM. Molybdenum. The elemental composition of human tissues and body fluids: a compilation of values for adults. New York: Verlag Chemie; 1978. [Google Scholar]
- 456.Bougle D, Bureau F, Foucault P, Duhamel J, Muller G, Drosdowsky M. Molybdenum content of term and preterm human milk during the first 2 months of lactation. Am J Clin Nutr. 1988;48(3):652–4. [DOI] [PubMed] [Google Scholar]
- 457.Todd GD, Keith SMS, Faroon O, Melanie Buser MPH, Ingerman L, Hard C, et al. Toxicological profile for molybdenum. Chapter 3: Toxicokinetics, susceptible populations, biomarkers, chemical interactions. Atlanta, GA: U.S. [Google Scholar]
- 458.Mendel RR, Kruse T. Cell biology of molybdenum in plants and humans. BBA-Mol Cell Res. 2012;1823(9):1568–79. [DOI] [PubMed] [Google Scholar]
- 459.Schwarz G, Mendel RR, Ribbe MW. Molybdenum cofactors, enzymes and pathways. Nature. 2009;460(7257):839–47. [DOI] [PubMed] [Google Scholar]
- 460.Schwarz G. Molybdenum cofactor and human disease. Curr Opin Chem Biol. 2016;31:179–87. 10.1016/j.cbpa.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 461.Huisingh J, Matrone G. Copper-molybdenum interactions with the sulfate-reducing system in rumen microorganisms. P Soc Exp Biol Med. 1972;139(2):518–21. [DOI] [PubMed] [Google Scholar]
- 462.Gutiérrez-González E, García-Esquinas E, de Larrea-Baz NF, Salcedo-Bellido I, Navas-Acien A, Lope V, et al. Toenails as biomarker of exposure to essential trace metals: a review. Environ Res. 2019;179(Pt A):108787. 10.1016/j.envres.2019.108787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, et al. Characterization of mammalian selenoproteomes. Science. 2003;300(5624):1439–43. 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 464.Rayman MP. Selenoproteins and human health: insights from epidemiological data. BBA-Gen Subjects. 2009;1790(11):1533–40. 10.1016/j.bbagen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 465.Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–68. 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 466.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30(4):829–34. 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 467.Weeks BS, Hanna MS, Cooperstein D. Dietary selenium and selenoprotein function. Med Sci Monit. 2012;18(8):RA127–32. 10.12659/msm.883258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.He Y, Xiang Y, Zhou Y, Yang Y, Zhang J, Huang H, et al. Selenium contamination, consequences and remediation techniques in water and soils: a review. Environ Res. 2018;164:288–301. 10.1016/j.envres.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 469.Risher J. Toxicological profile for selenium. Atlanta, Georgia: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2003. [PubMed] [Google Scholar]
- 470.Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: a review. Sci Total Environ. 2008;400(1):115–41. 10.1016/j.scitotenv.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 471.Burk RF, Hill KE. Regulation of selenium metabolism and transport. Annu Rev Nutr. 2015;35:109–34. 10.1146/annurev-nutr-071714-034250. [DOI] [PubMed] [Google Scholar]
- 472.Johnson CC, Fordyce FM, Rayman MP. Symposium on ‘Geographical and geological influences on nutrition’ Factors controlling the distribution of selenium in the environment and their impact on health and nutrition: Conference on ‘Over- and undernutrition: challenges and approaches.’ Proc Nutr Soc. 2010;69(1):119–32. [DOI] [PubMed] [Google Scholar]
- 473.Fairweather-Tait SJ, Collings R, Hurst R. Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr. 2010;91(5):1484S–S1491. 10.3945/ajcn.2010.28674J. [DOI] [PubMed] [Google Scholar]
- 474.Butler JA, Thomson CD, Whanger PD, Robinson MF. Selenium distribution in blood fractions of New Zealand women taking organic or inorganic selenium. Am J Clin Nutr. 1991;53(3):748–54. 10.1093/ajcn/53.3.748. [DOI] [PubMed] [Google Scholar]
- 475.Bügel S, Sandström B, Skibsted LH. Pork meat: a good source of selenium? J Trace Elem Med Biol. 2004;17(4):307–11. 10.1016/S0946-672X(04)80033-6. [DOI] [PubMed] [Google Scholar]
- 476.Bergdahl IA. Fractionation of soluble selenium compounds from fish using size-exclusion chromatography with on-line detection by inductively coupled plasma mass spectrometry. Analyst. 1999;124(10):1435–8. 10.1039/A904024B. [DOI] [PubMed] [Google Scholar]
- 477.Kotrebai M, Birringer M, Tyson JF, Block E, Uden PC. Selenium speciation in enriched and natural samples by HPLC-ICP-MS and HPLC-ESI-MS with perfluorinated carboxylic acid ionpairing agents. Analyst. 2000;125(1):71–8. 10.1039/A906320J. [DOI] [PubMed] [Google Scholar]
- 478.Kirby JK, Lyons GH, Karkkainen MP. Selenium speciation and bioavailability in biofortified products using species-unspecific isotope dilution and reverse phase ion pairing−inductively coupled plasma−mass spectrometry. J Water Pollut Control Fed. 2008;56(5):1772–9. 10.1021/jf073030v. [DOI] [PubMed] [Google Scholar]
- 479.Roman M, Jitaru P, Barbante C. Selenium biochemistry and its role for human health. Metallomics. 2014;6(1):25–54. 10.1039/c3mt00185g. [DOI] [PubMed] [Google Scholar]
- 480.Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem. 2007;282(16):12290–7. 10.1074/jbc.M611403200. [DOI] [PubMed] [Google Scholar]
- 481.Olson GE, Winfrey VP, Hill KE, Burk RF. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem. 2008;283(11):6854–60. 10.1074/jbc.M709945200. [DOI] [PubMed] [Google Scholar]
- 482.Suzuki Y, Hashiura Y, Matsumura K, Matsukawa T, Shinohara A, Furuta N. Dynamic pathways of selenium metabolism and excretion in mice under different selenium nutritional statuses. Metallomics. 2010;2(2):126–32. 10.1039/B915816B. [DOI] [PubMed] [Google Scholar]
- 483.Kobayashi Y, Ogra Y, Ishiwata K, Takayama H, Aimi N, Suzuki KT. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc Natl Acad Sci U S A. 2002;99(25):15932–6. 10.1073/pnas.252610699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Alexander J. Chapter 52 - Selenium. In: Nordberg GF, Fowler BA, Nordberg M, editors. Handbook on the toxicology of metals. 4th ed. San Diego: Academic Press; 2015. p. 1175–208. [Google Scholar]
- 485.Maehira F, Luyo GA, Miyagi I, Oshiro M, Yamane N, Kuba M, et al. Alterations of serum selenium concentrations in the acute phase of pathological conditions. Clin Chim Acta. 2002;316(1–2):137–46. [DOI] [PubMed] [Google Scholar]
- 486.Hill KE, Xia Y, Akesson B, Boeglin ME, Burk RF. Selenoprotein P concentration in plasma is an index of selenium status in selenium-deficient and selenium-supplemented Chinese subjects. J Nutr. 1996;126(1):138–45. 10.1093/jn/126.1.138. [DOI] [PubMed] [Google Scholar]
- 487.Isobe Y, Asakura H, Tsujiguchi H, Kannon T, Takayama H, Takeshita Y, et al. Alcohol intake is associated with elevated serum levels of selenium and selenoprotein P in humans. Front Nutr. 2021;8:633703. 10.3389/fnut.2021.633703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev. 2006;15(4):804–10. 10.1158/1055-9965.EPI-05-0950. [DOI] [PubMed] [Google Scholar]
- 489.Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7(4):384–90. 10.1097/00001648-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 490.Alfthan G. Can externally deposited Se be removed from hair? Clin Chem. 1985;31(3):500. [PubMed] [Google Scholar]
- 491.Hawkes WC, Alkan FZ, Oehler L. Absorption, distribution and excretion of selenium from beef and rice in healthy North American men. J Nutr. 2003;133(11):3434–42. 10.1093/jn/133.11.3434. [DOI] [PubMed] [Google Scholar]
- 492.Clavaud C, Michelin C, Pourhamidi S, Ziane S, El Rawadi C, Muller B, et al. Selenium disulfide: a key ingredient to rebalance the scalp microbiome and sebum quality in the management of dandruff. Eur J Dermatol. 2023;33(S1):5–12. 10.1684/ejd.2023.4400. [DOI] [PubMed] [Google Scholar]
- 493.Majeed M, Majeed S, Nagabhushanam K, Mundkur L, Neupane P, Shah K. Clinical study to evaluate the efficacy and safety of a hair serum product in healthy adult male and female volunteers with hair fall. Clin Cosmet Investig Dermatol. 2020;13:691–700. 10.2147/ccid.S271013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Lee AM, Huel G, Godin J, Hellier G, Sahuquillo J, Moreau T, et al. Inter-individual variation of selenium in maternal plasma, cord plasma and placenta. Sci Total Environ. 1995;159(2):119–27. 10.1016/0048-9697(95)04123-I. [DOI] [PubMed] [Google Scholar]
- 495.Lorenzo Alonso MJ, Bermejo Barrera A, de Juan JAC, Fraga Bermúdez JM, Bermejo BP. Selenium levels in related biological samples: human placenta, maternal and umbilical cord blood, hair and nails. J Trace Elem Med Biol. 2005;19(1):49–54. 10.1016/j.jtemb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 496.Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, Karagas MR. Placental metal concentrations in relation to maternal and infant toenails in a U.S. cohort. Environ Sci Technol. 2016;50(3):1587–94. 10.1021/acs.est.5b05316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Roney N. Toxicological profile for zinc. Atlanta, Georgia: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2005. [PubMed] [Google Scholar]
- 498.Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract. 2015;30(3):371–82. [DOI] [PubMed] [Google Scholar]
- 499.Part III. Minerals. Zinc. In: Meyers LD, Hellwig JP, Otten JJ, editors. Dietary reference intakes: the essential guide to nutrient requirements: National Academies Press; 2006. [Google Scholar]
- 500.Brown JL. Zinc fume fever. Brit J Radiol. 1988;61(724):327–9. [DOI] [PubMed] [Google Scholar]
- 501.Drinker KR, Drinker P. Metal Fume Fever: V. Results of the inhalation by animals of zinc and magnesium oxide fumes. J Ind Hyg. 1928;10:56–70. [Google Scholar]
- 502.Malo J, Malo J, Cartier A, Dolovich J. Acute lung reaction due to zinc inhalation. Eur Respir J. 1990;3(1):111–4. [PubMed] [Google Scholar]
- 503.Lombardi-Boccia G, Aguzzi A, Cappelloni M, Di Lullo G, Lucarini M. Total-diet study: dietary intakes of macro elements and trace elements in Italy. Brit J Nutr. 2003;90(6):1117–21. [DOI] [PubMed] [Google Scholar]
- 504.Roney N. Toxicological profile for zinc. Chapter 6: Potential for human exposure. Atlanta, Georgia: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2005. [Google Scholar]
- 505.Mirenda RJ. Acute toxicity and accumulation of zinc in the crayfish, Orconectes virilis (Hagen). B Environ Contam Tox. 1986;37(1):387–94. [DOI] [PubMed] [Google Scholar]
- 506.Fishbein L. Sources, transport and alterations of metal compounds: an overview. I. Arsenic, beryllium, cadmium, chromium, and nickel. Environ Health Persp. 1981;40:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Gerhardsson L, Englyst V, Lundström N-G, Sandberg S, Nordberg G. Cadmium, copper and zinc in tissues of deceased copper smelter workers. J Trace Elem Med Biol. 2002;16(4):261–6. [DOI] [PubMed] [Google Scholar]
- 508.Sharrett AR, Carter AP, Orheimt RM, Feinleib M. Daily intake of lead, cadmium, copper, and zinc from drinking water: the Seattle study of trace metal exposure. Environ Res. 1982;28(2):456–75. [DOI] [PubMed] [Google Scholar]
- 509.Pecoud A, Donzel P, Schelling J. Effect of foodstuffs on the absorption of zinc sulfate. Clin Pharmacol Ther. 1975;17(4):469–74. [DOI] [PubMed] [Google Scholar]
- 510.Nelson LS Jr, Jacobs FA, Brushmiller JG. Solubility of calcium and zinc in model solutions based on bovine and human milks. J Inorg Biochem. 1985;24(4):255–65. [DOI] [PubMed] [Google Scholar]
- 511.Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985;65(2):238–309. [DOI] [PubMed] [Google Scholar]
- 512.Wastney M, Aamodt R, Rumble W, Henkin R. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am J Physiol-Reg I. 1986;251(2):R398–408. [DOI] [PubMed] [Google Scholar]
- 513.Bentley P, Grubb B. Experimental dietary hyperzincemia tissue disposition of excess zinc in rabbits. Trace Elem Med. 1991;8(4):202–7. [Google Scholar]
- 514.Llobet J, Domingo J, Colomina M, Mayayo E, Corbella J. Subchronic oral toxicity of zinc in rats. B Environ Contam Tox. 1988;41(1):36–43. [DOI] [PubMed] [Google Scholar]
- 515.Liu-Sheng H, Xiao-Shan Y, De-Chang W. Age-dependent variation of zinc-65 metabolism in LACA mice. Int J Radiat Biol. 1991;60(6):907–16. [DOI] [PubMed] [Google Scholar]
- 516.Spencer H, Kramer L, Osis D. Zinc metabolism in man. J Environ Pathol Tox. 1985;5(6):265–78. [PubMed] [Google Scholar]
- 517.Alexander J, Aaseth J, Refsvik T. Excretion of zinc in rat bile–a role of glutathione. Acta Pharmacol Tox. 1981;49(3):190–4. [DOI] [PubMed] [Google Scholar]
- 518.Bandeira VDS, Pires LV, Hashimoto LL, Alencar LL, Almondes KGS, Lottenberg SA, et al. Association of reduced zinc status with poor glycemic control in individuals with type 2 diabetes mellitus. J Trace Elem Med Biol. 2017;44:132–6. 10.1016/j.jtemb.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 519.Lech T, Sadlik JK. Zinc in postmortem body tissues and fluids. Biol Trace Elem Res. 2011;142(1):11–7. 10.1007/s12011-010-8747-5. [DOI] [PubMed] [Google Scholar]
- 520.Honda R, Tawara K, Nishijo M, Nakagawa H, Tanebe K, Saito S. Cadmium exposure and trace elements in human breast milk. Toxicology. 2003;186(3):255–9. [DOI] [PubMed] [Google Scholar]
- 521.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr. 2009;89(6):2040S–S2051. 10.3945/ajcn.2009.27230G. [DOI] [PubMed] [Google Scholar]
- 522.Hennigar SR, Kelley AM, McClung JP. Metallothionein and zinc transporter expression in circulating human blood cells as biomarkers of zinc status: a systematic review. Adv Nutr. 2016;7(4):735–46. 10.3945/an.116.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Maret W. Zinc and Human Disease. In: Sigel A, Sigel H, Sigel RKO, editors. Interrelations between essential metal ions and human diseases. Metal Ions in Life Sciences. Dordrecht: Springer Netherlands; 2013. pp, 389–414. [DOI] [PubMed] [Google Scholar]
- 524.Hobisch-Hagen P, Mörtl M, Schobersberger W. Hemostatic disorders in pregnancy and the peripartum period. Acta Anaesth Scand Suppl. 1997;111:216–7. [PubMed] [Google Scholar]
- 525.Duncan A, Talwar D, McMillan DC, Stefanowicz F, O’Reilly DSJ. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr. 2012;95(1):64–71. [DOI] [PubMed] [Google Scholar]
- 526.Galloway SP, McMillan DC, Sattar N. Effect of the inflammatory response on trace element and vitamin status. Ann Clin Biochem. 2000;37(3):289–97. [DOI] [PubMed] [Google Scholar]
- 527.Taylor A. Detection and monitoring of disorders of essential trace elements. Ann Clin Biochem. 1996;33(6):486–510. [DOI] [PubMed] [Google Scholar]
- 528.Ruz M, Cavan KR, Bettger WJ, Gibson RS. Erythrocytes, erythrocyte membranes, neutrophils and platelets as biopsy materials for the assessment of zinc status in humans. Br J Nutr. 1992;68(2):515–27. 10.1079/bjn19920109. [DOI] [PubMed] [Google Scholar]
- 529.King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, et al. Biomarkers of Nutrition for Development (BOND)—zinc review. J Nutr. 2016;146(4):858S–S885. 10.3945/jn.115.220079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.McBean LD, Mahloudji M, Reinhold JG, Halsted JA. Correlation of zinc concentrations in human plasma and hair. Am J Clin Nutr. 1971;24(5):506–9. [DOI] [PubMed] [Google Scholar]
- 531.Rivlin RS. Misuse of hair analysis for nutritional assessment. Am J Med. 1983;75(3):489–93. [DOI] [PubMed] [Google Scholar]
- 532.Institute of Medicine (US) Subcommittee on interpretation and uses of dietary reference intakes. DRI dietary reference intakes: applications in dietary assessment. Washington (DC): National Academies Press (US); 2000. [PubMed] [Google Scholar]
- 533.Sommar JN, Hedmer M, Lundh T, Nilsson L, Skerfving S, Bergdahl IA (2014) Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J Expo Sci Environ Epidemiol. 24(1):51–7. 10.1038/jes.2013.4 [DOI] [PubMed] [Google Scholar]
- 534.Ansoborlo E, Lebaron-Jacobs L, Prat O. Uranium in drinkingwater: a unique case of guideline value increases and discrepancies between chemical and radiochemical guidelines. Environ Int. 2015;77:1–4. 10.1016/j.envint.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 535.Roney N, Colman J. Interaction profile for lead, manganese, zinc, and copper. Environ Toxicol Pharmacol. 2004;18(3):231–4. [DOI] [PubMed] [Google Scholar]
- 536.Weaver VM, Vargas GG, Silbergeld EK, Rothenberg SJ, Fadrowski JJ, Rubio-Andrade M, et al. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ Res. 2014;132:226–32. 10.1016/j.envres.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Abuawad A, Goldsmith J, Herbstman JB, Parvez F, Islam T, LoIacono N, et al. Urine dilution correction methods utilizing urine creatinine or specific gravity in arsenic analyses: comparisons to blood and water arsenic in the FACT and FOX studies in Bangladesh. Water. 2022;14(9):1477. 10.3390/w14091477. [DOI] [Google Scholar]
- 538.Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46(9):5118–25. 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As the manuscript is a narrative review, no data analysis was conducted and therefore, a data availability statement likely does not apply to this manuscript.


