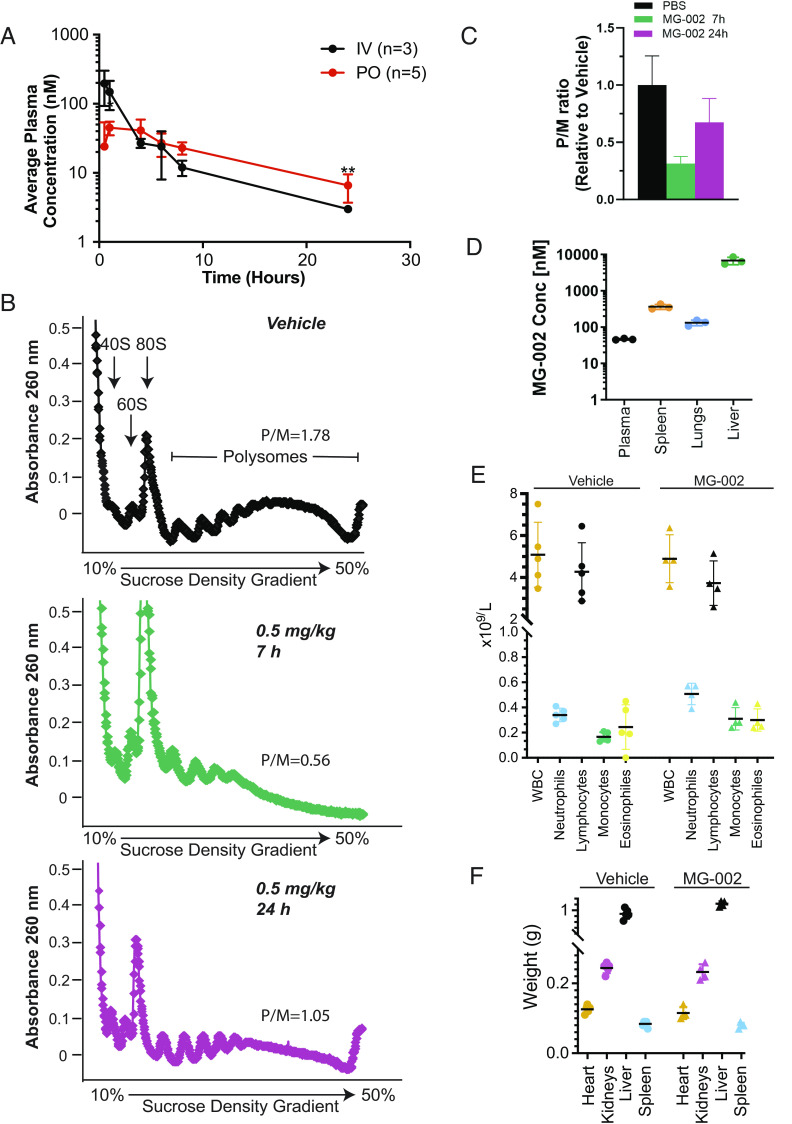
Fig. 4.
MG-002 accumulates in biological tissues and has minimal toxicities. (A) Average plasma concentration of MG-002 at the indicated time points following compound delivery by intravenous (IV) versus oral gavage (PO). (B) Polysomes isolated from livers of mice treated with vehicle or at the indicated time points following delivery of 0.5 mg/kg MG-002 PO. Polysome/monosome (P/M) ratio represents the average of two experiments. (C) Quantification of the P/M ratios shown in panel B. (D) Concentration of MG-002 in the indicated tissues 4 h after PO delivery of MG-002 (5 mg/kg) (n = 3 ±SD). (E) Total blood cell counts (total leukocytes, neutrophils, lymphocytes, monocytes, or eosinophils) from mice treated with vehicle or 0.5 mg/kg MG-002 PO on Mon/Wed/Fri for two consecutive weeks (n = 4 to 5 ±SD). (F) Tissue weights from mice treated with vehicle or 0.5 mg/kg MG-002 PO Mon/Wed/Fri for two consecutive weeks, after which time organs were harvested (n = 4 to 5 ±SD).