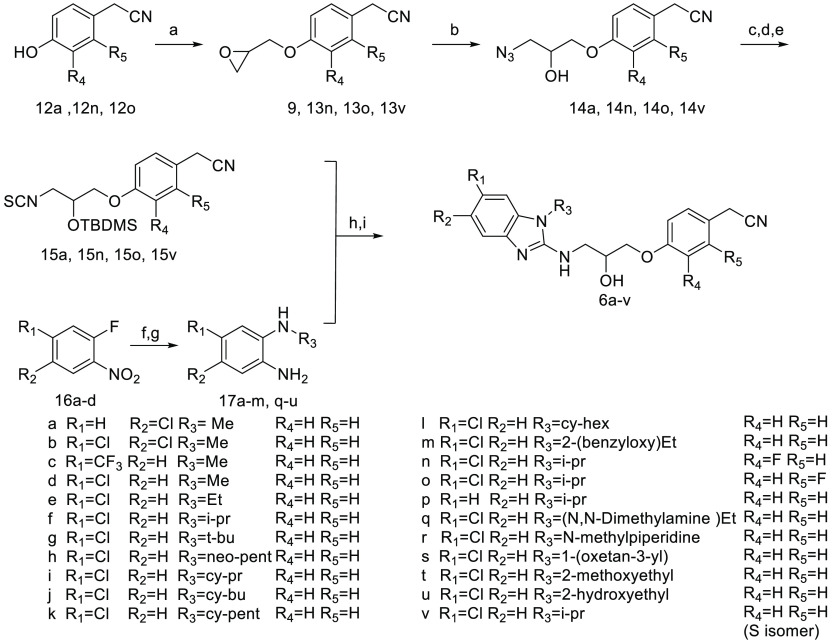
Scheme 2. Synthetic Route for the Preparation of Benzo[d]imidazole Analogues 2a–v.
Reagent and conditions: (a) eepichlorohydrin, Cs2CO3, CH3CN, reflux, 16 h, 25–28% or (S)-(−)-glycidyl nosylate for 13v, Cs2CO3, CH3CN, reflux, 16 h 85–87%; (b) NaN3, NH4Cl, EtOH 40 °C, 16 h, 95–100%; (c) TBDMS-Cl, imidazole, DMF, rt, 16 h, 75–84%; (d) PPh3, 10% H2O: THF, rt, 16 h 79–90%; (e) di(1H-imidazol-1-yl)methanethione, DCM, rt, 16 h 21–25%; (f) R3-NH2, MeOH, reflux, 16 h, 90–95%; (g) Zn, NH4Cl, 10% CH3COOH; MeOH, rt, 1 h, 90–95%; (h) EtOH, reflux, 16 h, then DIC, Et3N, DMF, reflux, 16 h, 50–65%; (i) 10% TFA; MeOH, rt, 48 h 53–60%.