Abstract
Background
Patients who require an emergency laparotomy suffer from high mortality and morbidity rates. Studies have shown that the standardization of perioperative management reduces complications in the short term. The aim of the present study was to report long-term mortality rates for the SMASH (Standardized perioperative Management of patients operated with acute Abdominal Surgery in a High-risk and emergency setting) study, as well as short- and long-term outcomes for different age groups within the SMASH study.
Methods
A prospective intervention study was introduced in 2018, with the aim of investigating the introduction of a standardized protocol for emergency laparotomy. For 42 months, intervention patients were managed according to the protocol and outcomes were then compared with those of historical controls.
Results
A total of 1344 unique patients were included (681 in the intervention group and 663 in the control group). The 90-day mortality rate was 14.1 per cent in the intervention group and 20.8 per cent in the control group (P = 0.002) and the 1-year mortality rate in adjusted analyses was 19.7 and 27.8 per cent respectively (P =< 0.001). An age-related subgroup analysis showed that the oldest patients (76 years and older, 260 in the intervention group and 240 in the control group) had a 1-year mortality rate of 29.6 and 43.8 per cent respectively (P = 0.004) and a mean duration of hospital stay of 9.9 and 11.6 days respectively (P = 0.027). Among older adults (61–75 years), the mean duration of hospital stay was 11.7 days in the intervention group compared with 15.1 days in the control group (P = 0.009) and the mean duration of ICU care was reduced to 4.49 days compared with 7.29 days (P = 0.046).
Conclusion
The standardized protocol associated with an emergency laparotomy appears to be beneficial, even in the long term. For elderly patients, it appears to reduce mortality rates and the durations of hospital stay and ICU care.
The SMASH (Standardized perioperative Management of patients operated with acute Abdominal Surgery in a High-risk and emergency setting) study is a prospective intervention study, introduced in 2018, with the aim of investigating the introduction of a standardized management protocol for emergency laparotomy. The results show that the postoperative 1-year mortality rate in adjusted analyses was 19.7 per cent in the intervention group and 27.8 per cent in the control group. The standardized clinical protocol appears to be beneficial for patients undergoing emergency laparotomy, even in the long term.
Introduction
Patients requiring surgery for an acute abdominal pathology—in most cases an emergency laparotomy—comprise a group of patients with among the highest mortality and complication rates in surgery1. The high incidence of short-term complications after emergency laparotomy is well known1–13. A patient is often critically ill due to the underlying condition and sepsis, and failure of one or more organ systems is common14. Patient management requires urgent and well-functioning cooperation between healthcare workers and sometimes resuscitation and critical care before anaesthesia15. Several studies in recent years have shown that a standardized perioperative protocol for patient management can reduce short-term mortality and complication rates7,10,12,16.
Long-term outcomes after an emergency laparotomy have also been investigated; several studies report a 1-year mortality rate of 25–34.1 per cent3,17,18. A systematic review from 2021 reports a 1-year mortality rate of 24.6 per cent from six published studies19. For older adults, a large retrospective US study of 468 000 patients, 65 years and older, also grading the patients according to frailty modelled on the Rockwood Frailty Index, shows a 1-year mortality rate of 21.6 per cent for patients categorized as non-frail and 53.7 per cent for patients categorized as moderately to severely frail20. A British single-centre study reports a 1-year mortality rate of 37 per cent for those over 70 years of age17. For the oldest group (90 years and older), a 1-year mortality rate of 68.6 per cent is reported3. Finally, in a systematic review with a geriatric subgroup from six studies (median age 79–85 years), a 1-year mortality rate of 30–47 per cent is reported21.
The Danish AHA (Acute High-risk Abdominal) study reports a 180-day mortality rate of 22.2 per cent in their intervention group and 29.5 per cent in the control group12. Besides that, there is a knowledge gap relating to how the intervention of standardized management affects long-term mortality rates after an emergency laparotomy.
The Swedish SMASH (Standardized perioperative Management of patients operated with acute Abdominal Surgery in a High-risk and emergency setting) controlled study previously presented the short-term postoperative outcomes after an emergency laparotomy16. The aim of the present study was to explore the impact of standardized management on long-term mortality and complication rates compared with a control group. Secondary aims were to explore mortality and complication rates in relation to different age groups.
Methods
The present study examined the secondary endpoints of the SMASH study16 (90-day and 1-year mortality rates, the need for intensive care, the duration of hospital stay, duration of ICU care and surgical complications according to the Clavien–Dindo scale22) for all patients.
In addition, subgroup analyses for four different age groups (18–40 years, 41–60 years, 61–75 years, and 76 years and older) were performed for all primary and secondary endpoints of the SMASH study population.
The study was approved by the Swedish Ethical Review Authority (reference number 868-17).
Patient selection
All patients in the present study underwent surgery at the NÄL County Hospital and the NU Hospital Group, County of Vastra Gotaland, Sweden.
Intervention group
All consecutive adult patients who underwent acute high-risk abdominal surgery, that is an emergency laparotomy and in selected cases a laparoscopy, with a priority to start surgery within 6 h or less from notification, were included in the intervention group23. The defined pathologies included in the present study were bowel obstruction or perforation, ischaemia, haemorrhage, surgical complication, or trauma laparotomy (Table 1).
Table 1.
Demographics and baseline characteristics for all individuals
| Variable | Intervention (n = 681) | Control (n = 663) | P |
|---|---|---|---|
| Age (years), mean(s.d.), median (range) | |||
| All individuals | 67.6(16.8), 71 (18–97) | 66.0(17.5), 69 (18–96) | 0.083 |
| Age group (years), mean(s.d.), median (range) | |||
| 18–40 | 30.9(6.6), 31.5 (18–40) (n = 56) | 30.4(6.5), 31 (18–40) (n = 66) | 0.744 |
| 41–60 | 52.3(5.6), 53 (41–60) (n = 149) | 51.2(5.6), 52 (41–60) (n = 152) | 0.095 |
| 61–75 | 69.0(4.3), 69.5 (61–75) (n = 216) | 68.5(4.1), 69 (61–75) (n = 205) | 0.223 |
| ≥76 | 83.1(5.2), 82 (76–97) (n = 260) | 83.0(4.7), 83 (76–96) (n = 240) | 0.792 |
| Sex | 0.755 | ||
| Male | 317 | 302 | |
| Female | 364 | 361 | |
| Co-morbidity | |||
| Chronic obstructive lung disease | 67 (9.8) | 54 (8.1) | 0.323 |
| Ischaemic heart disease | 95 (14.0) | 80 (12.1) | 0.345 |
| Congestive heart failure | 44 (6.5) | 59 (8.9) | 0.115 |
| Diabetes | 84 (12.3) | 76 (11.5) | 0.683 |
| Chronic renal failure | 26 (3.8) | 30 (4.5) | 0.609 |
| Obesity | 99 (14.5) | 82 (12.4) | 0.278 |
| Smoking | 80 (11.7) | 86 (13.0) | 0.549 |
| No co-morbidity | 357 (52.4) | 333 (50.2) | 0.453 |
| ASA classification | |||
| I | 48 (7.0) | 72 (10.9) | – |
| II | 254 (37.3) | 222 (33.5) | – |
| III | 280 (41.1) | 264 (39.8) | – |
| IV | 79 (11.6) | 94 (14.2) | – |
| V | 20 (2.9) | 11 (1.7) | 0.439 |
| Cancer | 199 (29.2) | 204 (30.8) | 0.581 |
| Diagnosis at surgery | |||
| Peritonitis | |||
| No peritonitis | 532 (78.1) | 489 (73.8) | – |
| Purulent | 38 (5.6) | 42 (6.3) | – |
| Faecal | 59 (8.7) | 48 (7.2) | – |
| Other | 52 (7.6) | 84 (12.7) | 0.015 |
| Intestinal ischaemia | 91 (13.4) | 76 (11.5) | 0.331 |
| Bowel obstruction | |||
| No obstruction | 273/677 (40.3) | 277/659 (42.0) | – |
| Small intestine | 304/677 (44.9) | 314/659 (47.6) | – |
| Colon | 100/677 (14.8) | 68/659 (10.3) | 0.049 |
| Trauma | 15 (2.2) | 20/661 (3.0) | 0.439 |
| Bleeding | 33 (4.8) | 41/659 (6.2) | 0.326 |
| Perforation | |||
| No perforation | 469/675 (69.5) | 460 (69.4) | – |
| Colon | 87/675 (12.9) | 57 (8.6) | – |
| Small intestine | 62/675 (9.2) | 72 (10.9) | – |
| Stomach | 37/675 (5.5) | 56 (8.4) | – |
| Anastomosis | 20/675 (3.0) | 18 (2.7) | 0.027 |
Values are n (%) unless otherwise indicated. For comparisons between groups, Fisher’s exact test (lowest one-sided P value multiplied by two) was used for dichotomous variables, the Mantel–Haenszel chi-squared test was used for ordered categorical variables, the chi-squared test was used for non-ordered categorical variables, and Fisher’s non-parametric permutation test was used for continuous variables.
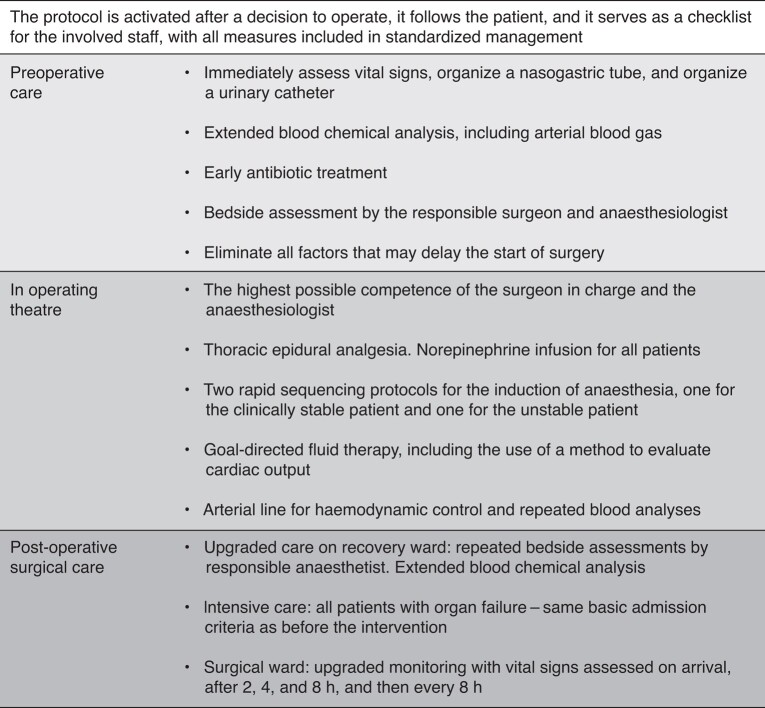
The SMASH care bundle (Fig. 1) consists of several actions in the form of a clinical protocol that is activated after a decision to operate, follows the patient until hospital discharge, and serves as a checklist for the healthcare workers involved.
Fig. 1.
Main actions of the SMASH care bundle for emergency laparotomy
Vital signs, that is early warning score: heart rate, blood pressure, respiratory rate, oxygen saturation, level of consciousness, and body temperature. Extended blood chemical analyses: haemoglobin concentration, platelet count, white blood cell count, sodium concentration, potassium concentration, creatinine kinase concentration, C-reactive protein concentration, procalcitonin concentration, and arterial blood gas levels.
The most important elements of the care bundle can be divided into three phases. First, the preoperative phase, where the actions are focused on assessing the clinical condition of the patient, starting antimicrobial treatment, and accomplishing well-functioning, speedy cooperation between the clinicians involved. Second, the phase in the operating theatre, where the standardization aims to ensure a high level of clinical competence and good planning in surgical and anaesthesiological interventions and a high level of intraoperative patient monitoring. Last, the postoperative phase, where care in recovery is upgraded, with extended blood chemical analyses and bedside assessments by the responsible anaesthetist. The criteria for postoperative admission to the ICU were the same for the control group and the intervention group and were not changed during the study interval. The concept used as admission criteria for ICU in the Swedish context is based on the clinical status and organ function of the patient at any given time. On the regular surgical ward, there is extra emphasis on monitoring vital signs, to be able to detect at an early stage whether a patient is deteriorating. The SMASH care bundle has also been described in previously published manuscripts16,23 and the original standardized clinical protocol in Swedish is available in the Supplementary material.
Control group
The retrospectively collected control group consisted of consecutive patients who underwent high-risk abdominal surgery during the interval 20 August 2014 to 20 October 2017. The indication for surgery was the same as in the intervention group, that is the inclusion and exclusion criteria were the same. Standardized management was not introduced for emergency laparotomies during this interval and all patient-related decisions and clinical strategies were determined by the responsible surgeons and anaesthetists.
Missed cases
In cases where, upon discharge from hospital, it was found that the standardized protocol had not been implemented, the patient was not included in the intervention group and the patient was then regarded as a missed case16.
Variables
Demographic and clinical data were collected by reviewing the included patients’ computerized medical records (Melior©), as well as the surgical operation planning system (Orbit©). The incidence of cancer was defined by whether the specific individual had received a cancer diagnosis in hospital care during a follow-up interval of 3 years before until 1 year after the time of surgery. All cancer diagnoses made in hospital were included, except for basal cell carcinomas (diagnosis code C-44).
The source of management data was the computerized medical record systems and, in the case of the intervention group, the activated protocol for standardized management. Two different kinds of outcome data were collected (data on surgical complications and time-point data (including all dates and times for the entire intervention)). Management time points were collected from computerized medical records (Melior©), as well as the surgical operation planning system (Orbit©), and mortality rate follow-up was performed using the Swedish Population Register, by carrying out a search using each patient’s unique personal code number.
Data and statistical analyses
On inclusion, each patient’s medical record data were scrutinized in both groups. During this phase of processing and analysing, all the data were de-identified. For categorical variables, numbers with percentages are presented. The adjusted OR was analysed by GENMOD24 (General Mode) with the generalized estimated equation (GEE) model with binary outcome and link function logit adjusted for age, intestinal ischaemia, faecal/purulent/other peritonitis, chronic obstructive lung disease, ischaemic heart disease, congestive heart failure, chronic renal failure, diabetes, obesity, smoking, ASA classification, sex, and cancer. The OR was analysed by GENMOD with the GEE model with binary outcome and link function logit. For comparisons between groups, Fisher’s exact test (lowest one-sided P value multiplied by two) was used for dichotomous variables. The confidence interval for dichotomous variables was the asymptotic Wald confidence limits with continuity correction. Fisher’s non-parametric permutation test for continuous variables and the chi-squared test were used for unordered categorical variables. Statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA).
Results
Long-term results
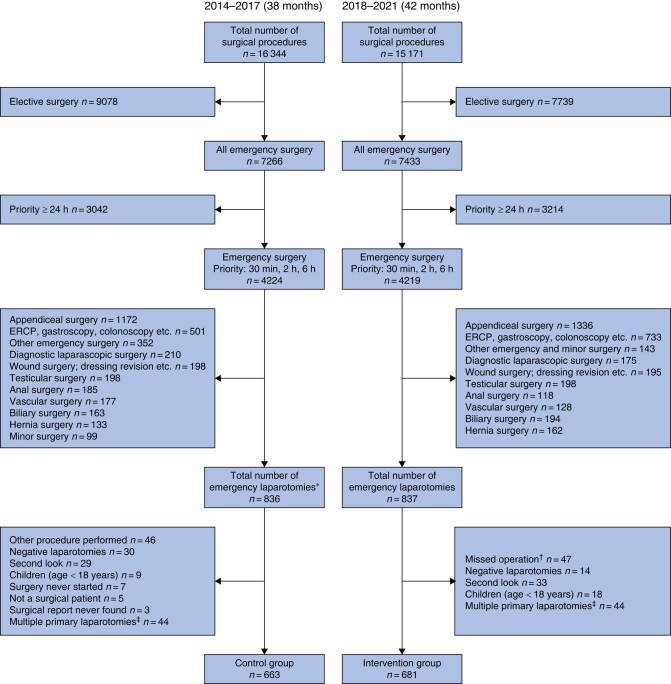
A total of 1344 patients were included in the present study, in the interval from 2014 to 2021 (38 months in 2014–2017 for the control group and 42 months in 2018–2021 for the intervention group) (Fig. 2). The median age of patients in the intervention group was 71 years, whereas that of patients in the control group was 69 years. The most common physical status grade was ASA III in both groups (41.1 per cent in the intervention group and 39.8 per cent in the control group). An existing diagnosis of cancer was identified during the follow-up interval for 29.2 per cent of patients in the intervention group and 30.8 per cent of patients in the control group. No significant differences in demographics were seen (Table 1).
Fig. 2.
Study structure for the control and intervention groups
*Including laparotomies that had a different procedure code initially. †Standardized protocol never used. ‡Each individual can only be included once. ERCP, endoscopic retrograde cholangioapancreatography.
Perioperative treatment with antibiotics occurred in 94.7 per cent of patients in the intervention group and 81.4 per cent of patients in the control group. A higher proportion of patients in the intervention group received epidural anaesthesia (71.1 per cent) compared with patients in the control group (67.0 per cent) (Table 2). No difference was seen in the total number of bowel resections and stoma formations, but more surgery on the small intestine was performed in the intervention group and almost 6 per cent fewer anastomoses were carried out in the intervention group (Table 2).
Table 2.
Intervention variables for all individuals
| Variable | Intervention (n = 681) | Control (n = 663) |
|---|---|---|
| Management variables—preoperative | ||
| Antibiotics | 645 (94.7) | 524/644 (81.4) |
| Management variables—anaesthesiological | ||
| Epidural | 484 (71.1) | 444 (67.0) |
| Arterial line | 578 (84.9) | 244 (36.8) |
| Norepinephrine | 586/679 (86.3) | 474 (71.5) |
| Rapid sequence intubation | ||
| Propofol | 501 (79.0) | 550 (83.5) |
| Ketamine | 61 (9.6) | 60 (9.1) |
| Propofol + ketamine | 72 (11.4) | 49 (7.4) |
| Missing | 47 | 4 |
| Goal-directed fluid therapy | 527/679 (77.6) | * |
| Anaesthesia complication | ||
| No complication | 601 (91.6) | 573 (87.2) |
| Yes, aspiration | 11 (1.7) | 5 (0.8) |
| Yes, other complication | 44 (6.7) | 79 (12.0) |
| Missing | 25 | 6 |
| Postoperative care | ||
| Recovery unit, time spent (h), mean(s.d.), median (range) | 6.85(4.51), 5.22 (1.53–26.85) (n = 563) | 7.64(4.67), 5.87 (0.43–27.07) (n = 545) |
| Time-point variables | ||
| Degree of urgency | ||
| Emergency | 24 (3.5) | 23 (3.5) |
| Within 2 h | 372 (54.6) | 254 (38.3) |
| Within 6 h | 285 (41.9) | 386 (58.2) |
| Time from registration to the start of surgery (h), mean(s.d.), median (range) | 3.22(1.96), 2.73 (−0.52–17.3) (n = 681) | 3.80(3.36), 3.03 (0.08–54.12) (n = 663) |
| Total surgery time (min), mean(s.d.), median (range) | 94.0(48.2), 84 (12–335) (n = 681) | 90.7(48.8), 81 (20–375) (n = 663) |
| Perioperative care competence | ||
| Surgical | ||
| Registrar | 34/675 (5.0) | 56/660 (8.5) |
| Specialist | 177/675 (26.2) | 183/660 (27.7) |
| Consultant | 464/675 (68.7) | 421/660 (63.8) |
| Anaesthesiologist | ||
| Registrar | 207/679 (30.5) | 253/658 (38.4) |
| Specialist | 155/679 (22.8) | 127/658 (19.3) |
| Consultant | 317/679 (46.7) | 278/658 (42.2) |
| Surgical procedures | ||
| Primary operation | 602 (88.4) | 572 (86.3) |
| Reoperation | 79 (11.6) | 91 (13.7) |
| Initial laparoscopy | 45 (6.6) | * |
| Bowel resection | ||
| Any bowel resection | 240 (35.2) | 239 (36.0) |
| Type of resection | ||
| Colon | 117 (49.0) | 142 (59.4) |
| Small intestine | 110 (46.0) | 83 (34.7) |
| Colon and small intestine | 12 (5.0) | 14 (5.9) |
| Missing | 1 | 0 |
| Anastomosis | 114/679 (16.8) | 149 (22.5) |
| Stoma formation | 165/679 (24.3) | 169 (25.5) |
| Adhesiolysis | 314/678 (46.3) | 299 (45.1) |
| Extirpation organ | ||
| None | 369 (98.2) | 648 (97.7) |
| Part of/the whole stomach | 3 (0.4) | 4 (0.6) |
| Spleen | 5 (0.7) | 5 (0.8) |
| Part of liver | 0 (0.0) | 2 (0.3) |
| Uterus and/or ovaries | 3 (0.4) | 3 (0.5) |
| Other | 1 (0.1) | 1 (0.2) |
Values are n (%) unless otherwise indicated. *Not available for controls.
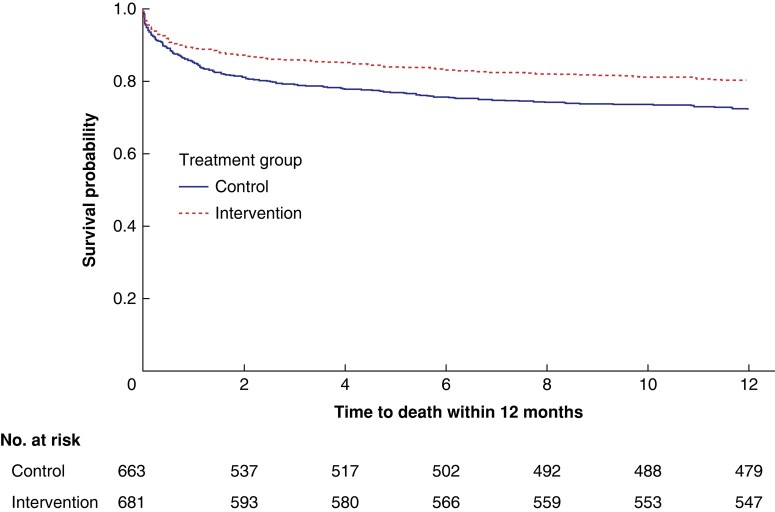
In adjusted analyses, the 90-day mortality rate for the patients in the SMASH study was 14.1 per cent in the intervention group and 20.8 per cent in the control group (P = 0.002). The 1-year mortality rate was 19.7 and 27.8 per cent respectively (P < 0.001) (Table 3 and Fig. 3). Previously presented short-term data from the SMASH study showed that the mean duration of hospital stay was 10.2 days in the intervention group and 11.9 days in the control group (P = 0.009). A mean reduction of 2.28 days in duration of ICU care was seen (mean duration of ICU care was 3.12 days in the intervention group compared with 5.4 days in the control group; P = 0.007) and, finally, a reduced percentage of serious surgical complications (Clavien–Dindo IIIb–V) was seen in the intervention group (27.3 per cent) compared with the control group (37.6 per cent) (Clavien-Dindo IIIb–IVb P = 0.024 and Clavien-Dindo V P = 0.002).
Table 3.
Endpoint analyses adjusted for all individuals
| Variable | Intervention (n = 681) | Control (n = 663) | GEE adjusted difference between groups (95% c.i.)/P | Difference between groups, mean (95% c.i.)/P |
|---|---|---|---|---|
| Death within 3 months | 96 (14.1) | 138 (20.8) | OR 1.69 (1.22,2.35)/0.002 | 6.7 (2.5,10.9)/0.002 |
| Death within 12 months | 134 (19.7) | 184 (27.8) | OR 1.70 (1.26,2.28)/<0.001 | 8.1 (3.4,12.8)/<0.001 |
| Duration of hospital stay (days), mean(s.d.), median (range) | 10.2(13.3), 7 (0–175.6) (n = 681) | 11.9(13.0), 7.5 (0.1–112.9) (n = 663) | LS mean −1.81 (−3.18,−0.45)/0.009 | −1.71 (−3.13,−0.31)/0.017 |
| ICU care | 133 (19.5) | 145 (21.9) | OR 1.19 (0.88,1.60)/0.26 | 2.3 (–2.1,6.8)/0.32 |
| Duration of ICU care (days), mean(s.d.), median (range) | 3.12(5.97), 1.29 (0.02–53.54) (n = 133) | 5.40(8.34), 2.1 (0.03–62.02) (n = 145) | LS mean −2.36 (−4.08,−0.65)/0.007 | −2.28 (−4.01,−0.59)/0.006 |
| Readmission to the ICU | 22 (3.2) | 30 (4.5) | OR 1.63 (0.91,2.93)/0.10 | 1.3 (−0.9,3.5)/0.28 |
| Surgical complications | – | – | LS mean −0.16 (−0.23,−0.09)/<0.001 | 0.0001 |
| No complications | 1 (0.1) | 7 (1.1) | – | 0.9 (−0.1; 1.9)/0.064 |
| Clavien–Dindo I–IIIa | 494 (72.5) | 407 (61.4) | OR 0.53 (0.41−0.69)/<0.001 | −11.2 (−16.3; −6.0)/<0.001 |
| Clavien–Dindo IIIb–IVb | 115 (16.9) | 141 (21.3) | OR 1.39 (1.04−1.84)/0.024 | 4.4 (0.0; 8.7)/0.048 |
| Clavien–Dindo V | 71 (10.4) | 108 (16.3) | OR 1.80 (1.25−2.59)/0.002 | 5.9 (2.1; 9.6)/0.002 |
Values are n (%) unless otherwise indicated. The adjusted OR was analysed by GENMOD (General Mode) with the generalized estimating equation (GEE) model with binary outcome and link function logit adjusted for age, intestinal ischaemia, faecal/purulent/other peritonitis, chronic obstructive lung disease, ischaemic heart disease, congestive heart failure, chronic renal failure, diabetes, obesity, smoking, ASA classification, sex, and cancer. The OR was analysed by GENMOD with the GEE model with binary outcome and link function logit. For comparisons between groups, Fisher’s exact test (lowest one-sided P value multiplied by two) was used for dichotomous variables. The confidence interval for dichotomous variables was the asymptotic Wald confidence limits with continuity correction. LS, Least Squares.
Fig. 3.
Kaplan–Meier survival curves for the control and intervention groups 1 year after surgery
Results from the age-group analysis
Demographic data for the different age groups (Table 4) showed that the largest age group was the 76 years and older group, which comprised 500 patients. The highest rates of peritonitis (28.7 per cent) and perforation (38.3 per cent) were seen in the intervention group for the 61–75 years age group. No significant differences in ASA classification were seen in the different age groups (Table S1).
Table 4.
Demographic and intervention variables for different age groups
| Age group (years) | Intervention (n = 681) |
Control (n = 663) |
P |
|---|---|---|---|
| 18–40 | n = 56 | n = 66 | |
| Sex | 1.000 | ||
| Male | 23 | 28 | |
| Female | 33 | 38 | |
| Primary operation | 51 (91.1) | 60 (90.9) | 1.000 |
| Reoperation | 5 (8.9) | 6 (9.1) | 1.000 |
| Co-morbidity | 14 (25.0) | 25 (37.9) | 0.184 |
| Cancer | 2 (3.6) | 1 (1.5) | 0.876 |
| Peritonitis | 8 (14.3) | 10 (15.2) | 1.000 |
| Ileus | 34 (60.7) | 28/65 (43.1) | 0.079 |
| Perforation | 9/54 (16.7) | 16 (24.2) | 0.431 |
| Bowel resection | 17 (30.4) | 12 (18.2) | 0.174 |
| Anastomosis | 13 (23.2) | 6 (9.1) | 0.058 |
| Stoma | 4 (7.1) | 6 (9.1) | 0.959 |
| Adhesions | 24 (42.9) | 19 (28.8) | 0.153 |
| Antibiotics | 52 (92.9) | 41/64 (64.1) | <0.001 |
| Epidural | 38 (67.9) | 40 (60.6) | 0.522 |
| Time from registration to the start of surgery (h), mean(s.d.), median (range) | 2.66(1.49), 2.47 (0.5–8.02) | 2.70(1.90), 2.46 (0.08–11.57) | 0.902 |
| 41–60 | n = 149 | n = 152 | |
| Sex | 0.607 | ||
| Male | 75 | 71 | |
| Female | 74 | 81 | |
| Primary operation | 133 (89.3) | 128 (84.2) | 0.262 |
| Reoperation | 16 (10.7) | 24 (15.8) | 0.262 |
| Co-morbidity | 64 (43.0) | 67 (44.1) | 0.936 |
| Cancer | 34 (22.8) | 29 (19.1) | 0.512 |
| Peritonitis | 31 (20.8) | 38 (25.0) | 0.467 |
| Ileus | 81/148 (54.7) | 88 (58.3) | 0.616 |
| Perforation | 46/148 (31.1) | 43 (28.3) | 0.687 |
| Bowel resection | 36 (24.2) | 43 (28.3) | 0.495 |
| Anastomosis | 21 (14.1) | 29 (19.1) | 0.314 |
| Stoma | 31 (20.8) | 23 (15.1) | 0.257 |
| Adhesions | 57 (38.3) | 67 (44.1) | 0.363 |
| Antibiotics | 140 (94.0) | 111/146 (76.0) | <0.001 |
| Epidural | 109 (73.2) | 109 (71.7) | 0.880 |
| Time from registration to the start of surgery (h), mean(s.d.), median (range) | 3.16(1.89), 2.78 (0.8–17.3) | 3.45(2.74), 2.65 (0.08–23.22) | 0.303 |
| 61–75 | n = 216 | n = 205 | |
| Sex | 0.883 | ||
| Male | 107 | 104 | |
| Female | 109 | 101 | |
| Primary operation | 180 (83.3) | 168 (82.0) | 0.806 |
| Reoperation | 36 (16.7) | 37 (18.0) | 0.806 |
| Co-morbidity | 107 (49.5) | 103 (50.2) | 0.962 |
| Cancer | 72 (33.3) | 79 (38.5) | 0.312 |
| Peritonitis | 62 (28.7) | 61 (29.8) | 0.896 |
| Ileus | 115/215 (53.5) | 119/204 (58.3) | 0.368 |
| Perforation | 82/214 (38.3) | 68 (33.2) | 0.319 |
| Bowel resection | 80 (37.0) | 85 (41.5) | 0.407 |
| Anastomosis | 33 (15.3) | 54 (26.3) | 0.006 |
| Stoma | 61 (28.2) | 54 (26.3) | 0.744 |
| Adhesions | 102 (47.4) | 112 (54.6) | 0.169 |
| Antibiotics | 205 (94.9) | 173/203 (85.2) | 0.001 |
| Epidural | 151 (69.9) | 138 (67.3) | 0.640 |
| Time from registration to the start of surgery (h), mean(s.d.), median (range) | 3.13(1.99), 2.63 (−0.52–13.05) | 4.03(4.50), 3.05 (0.17–54.12) | 0.003 |
| ≥76 | n = 260 | n = 240 | |
| Sex | 0.747 | ||
| Male | 112 | 99 | |
| Female | 148 | 141 | |
| Primary operation | 238 (91.5) | 216 (90.0) | 0.659 |
| Reoperation | 22 (8.5) | 24 (10.0) | 0.659 |
| Co-morbidity | 139 (53.5) | 135 (56.3) | 0.592 |
| Cancer | 91 (35.0) | 95 (39.6) | 0.334 |
| Peritonitis | 48 (18.5) | 65 (27.1) | 0.028 |
| Ileus | 174/258 (67.4) | 147/239 (61.5) | 0.198 |
| Perforation | 69/259 (26.6) | 76 (31.7) | 0.256 |
| Bowel resection | 106/259 (40.9) | 99 (41.3) | 1.000 |
| Anastomosis | 47/258 (18.2) | 60 (25.0) | 0.064 |
| Stoma | 69/258 (26.7) | 86 (35.8) | 0.036 |
| Adhesions | 131/258 (50.8) | 101 (42.1) | 0.064 |
| Antibiotics | 248 (95.4) | 199/231 (86.1) | <0.001 |
| Epidural | 186 (71.5) | 157 (65.4) | 0.169 |
| Time from registration to the start of surgery (h), mean(s.d.), median (range) | 3.45(2.03), 2.83 (0.65–16) | 4.12(2.79), 3.45 (0.5–25.55) | 0.004 |
Values are n (%) or n/n (%) unless otherwise indicated. For comparisons between groups, Fisher’s exact test (lowest one-sided P value multiplied by two) was used for dichotomous variables and the Mann–Whitney U test was used for continuous variables.
Management with perioperative antibiotics and thoracic epidural analgesia was more common for the patients in the intervention group for all of the age groups. The time from registration to the start of surgery was reduced in the intervention group for all of the age groups. Fewer anastomoses were carried out in the intervention group for the age groups of 61–75 years and 76 years and older (Table 4).
The age-related postoperative outcomes are presented in Table 5. For the 61–75 years age group, the mean duration of hospital stay was reduced from 15.1 days in the control group to 11.7 days in the intervention group (P = 0.009) and the mean duration of ICU care was reduced from 7.29 days in the control group to 4.49 days in the intervention group (P = 0.046). For the 76 years and older age group, the 90-day mortality rate was 22.3 per cent in the intervention group and 34.2 per cent in the control group (P = 0.008) and the 1-year mortality rate was 29.6 and 43.8 per cent respectively (P = 0.004). Also, the mean duration of hospital stay decreased from 11.6 days in the control group to 9.88 days in the intervention group (P = 0.016).
Table 5.
Endpoint analyses adjusted for different age groups
| Age group (years) | Intervention (all individuals n = 681) | Control (all individuals n = 663) | GEE adjusted difference between groups (95% c.i.)/P |
|---|---|---|---|
| 18–40 | n = 56 | n = 66 | |
| Death within 30 days | 0 (0.0) | 3 (4.5) | – |
| Death within 3 months | 0 (0.0) | 4 (6.1) | – |
| Death within 12 months | 2 (3.6) | 4 (6.1) | – |
| Duration of hospital stay (days), mean(s.d.), median (range) | 7.55(16.97), 4.07 (1.03–128.87) | 6.89(12.24), 4.14 (0.14–96.04) | LS mean 1.14 (−3.69,5.96)/0.64 |
| Duration of ICU care (days), mean(s.d.), median (range) | 1.04(0.52), 0.89 (0.56–1.92) (n = 5) | 6.01(8.56), 2.3 (0.07–25.99) (n = 9) | LS mean −1.35 (−4.88,2.17)/0.45 |
| 41–60 | n = 149 | n = 152 | |
| Death within 30 days | 7 (4.7) | 7 (4.6) | OR 1.58 (0.43,5.90)/0.49 |
| Death within 3 months | 11 (7.4) | 12 (7.9) | OR 0.86 (0.33,2.26)/0.76 |
| Death within 12 months | 15 (10.1) | 21 (13.8) | OR 1.49 (0.64,3.48)/0.35 |
| Duration of hospital stay (days), mean(s.d.), median (range) | 9.35(13.56), 5.4 (1–102.42) | 10.1(11.5), 6.5 (0.9–86.5) | LS mean −0.93 (−3.66,1.80)/0.50 |
| Duration of ICU care (days), mean(s.d.), median (range) | 2.81(2.64), 1.74 (0.76–11.23) (n = 18) | 6.73(7.46), 3.49 (0.42–29.23) (n = 27) | LS mean −4.06 (−7.59,−0.52)/0.027 |
| 61–75 | n = 216 | n = 205 | |
| Death within 30 days | 20 (9.3) | 26 (12.7) | OR 0.70 (0.34,1.42)/0.32 |
| Death within 3 months | 27 (12.5) | 40 (19.5) | OR 1.70 (0.91,3.18)/0.10 |
| Death within 12 months | 40 (18.5) | 54 (26.3) | OR 1.58 (0.92,2.73)/0.10 |
| Duration of hospital stay (days), mean(s.d.), median (range) | 11.7(17.1), 7.3 (0.2–175.6) | 15.1(16.9), 8.3 (0.5–112.9) | LS mean −4.10 (−7.18,−1.02)/0.009 |
| Duration of ICU care (days), mean(s.d.), median (range) | 4.49(8.57), 1.42 (0.02–53.54) (n = 55) | 7.29(11.04), 2.54 (0.23–62.02) (n = 57) | LS mean −3.79 (−7.52,−0.07)/0.046 |
| ≥76 | n = 260 | n = 240 | |
| Death within 30 days | 46 (17.7) | 60 (25.0) | OR 0.69 (0.42,1.13)/0.14 |
| Death within 3 months | 58 (22.3) | 82 (34.2) | OR 1.84 (1.18,2.87)/0.008 |
| Death within 12 months | 77 (29.6) | 105 (43.8) | OR 1.84 (1.21,2.78)/0.004 |
| Duration of hospital stay (days), mean(s.d.), median (range) | 9.88(7.19), 8.67 (0.04–44.48) | 11.6(9.0), 9.8 (0.1–47) | LS mean −1.61 (−3.03,−0.18)/0.027 |
| Duration of ICU care (days), mean(s.d.), median (range) | 2.04(2.82), 1.02 (0.02–17.67) (n = 55) | 2.53(3.08), 1.1 (0.03–12.82) (n = 52) | LS mean −0.15 (−1.31,1.02)/0.81 |
Values are n (%) unless otherwise indicated. The adjusted OR was analysed by GENMOD (General Mode) with the generalized estimated equation (GEE) model with binary outcome and link function logit adjusted for age, intestinal ischaemia, faecal/purulent/other peritonitis, chronic obstructive lung disease, ischaemic heart disease, congestive heart failure, chronic renal failure, diabetes, obesity, smoking, ASA classification, sex, and cancer. The OR was analysed by GENMOD with the GEE model with binary outcome and link function logit. For comparisons between groups, Fisher’s exact test (lowest one-sided P value multiplied by two) was used for dichotomous variables. The confidence interval for dichotomous variables was the asymptotic Wald confidence limits with continuity correction. LS, Least Squares.
Discussion
The main purpose of this publication regarding the SMASH study was to explore how standardized perioperative management affects the long-term mortality rates of adults undergoing an emergency laparotomy and, secondarily, to investigate the impact on outcomes for age-related subgroups. Significantly lower 90-day and 1-year mortality rates, as well as shorter durations of hospital stay and ICU care, were found in the intervention group compared with patients in the control group.
It is a widely accepted fact that high-risk acute abdominal surgery accounts for most complications in the field of acute surgery and prior research has thoroughly documented short- and long-term mortality rates6,13,17,19,21,25–27. Studies have also reported on how standardized management is able to reduce mortality rates in the short term7,10,12. However, the way long-term mortality rates are affected by perioperative standardized management has not been studied to the same extent. The present study reports an almost 29 per cent reduction in the 1-year mortality rate (19.7 per cent in the intervention group and 27.8 per cent in the control group), lower than in other unselected groups of patients after an emergency laparotomy3,8,9,17,18. This indicates that standardized management is beneficial to patients over time and suggests that the overall effect on mortality rates goes beyond the previously reported 30 days. In fact, data presented here show that the decrease in mortality rates continues for many months after surgery.
In an effort to explore the postoperative outcomes after an emergency laparotomy further, the entire cohort is divided into four age groups (young, 18–40 years; middle-aged, 41–60 years; older adults, 61–75 years; and the elderly, 76 years and older). However, in this division, several methodological problems arise. There is no established way of age-classifying surgical patients and so the SMASH study group pragmatically divided the cohort into smaller subgroups and attempted to achieve a reasonable group size and relevant division by age. Although the division method is not established, little research has been conducted to show how protocol-based management affects general outcomes for different age groups.
The SMASH study presents a decrease in the long-term mortality rate of 32 per cent and a reduction in duration of hospital stay of 15 per cent for patients aged 76 years and older. This is a larger reduction in duration of hospital stay than in previously presented studies, but there are many potential differences between groups and it is necessary to be cautious about drawing conclusions from such comparisons17,21. Furthermore, patients in the 61–75 years age group had a significantly shorter duration of hospital stay (11.7 days in the intervention group and 15.1 days in the control group) and reduced mortality rates and a shorter duration of ICU care are also seen. In fact, all the study endpoints are improved in the age-group analysis, apart from duration of hospital stay for the youngest and 30-day mortality for the middle-aged. However, the two youngest age groups represent very few individuals and no definitive conclusions can be drawn from these results. Convincing data show that increased age is associated with poorer outcomes8,21. It has previously been shown that geriatric competence is important in care28 and this is therefore also recommended in the care of the elderly who are about to undergo an emergency laparotomy29. Standardized care has been shown to be beneficial to the elderly when mortality rates up to 3 months are evaluated7. In a Canadian before-and-after-study by Khadaroo et al.30, an EASE (Elder-Friendly Approaches to the Surgical Environment) model (including patient-oriented rehabilitation, geriatric assessment, and early-discharge planning) was successfully implemented for a total of 684 patients aged 65 years and older undergoing emergency general surgery; the study demonstrates a significant reduction in major surgical complications. All the above findings indicate that standardized protocols may play a more important role in the management of older adults and the elderly undergoing acute high-risk abdominal surgery, even in the long term.
All standardizations of care that are introduced as care bundles struggle with the same problem, that is it is difficult to identify whether a single measure is more important than any other, and the SMASH care bundle is no exception. Regarding the complexity of the included measures, the overall goal was to improve the care that was achieved for the variables analysed in the standardization, but not for any specific variable. There are no major differences between the groups regarding primary laparotomy or reoperations. Other measures that have changed are outside the standardization and one of them, the intestinal anastomosis procedure, decreased for all subgroups, except the youngest, indicating that a damage-control surgical approach might have been used, which is known to improve outcomes for the critically ill31. Furthermore, fewer patients among those aged 76 years and older in the intervention group had perioperative peritonitis, even if the analysed data are adjusted for peritonitis, and this pathology is known to be associated with poorer outcomes.
One limitation of the present study is the long inclusion interval of about 7 years. An alternative solution would be to involve several surgical centres and conduct the project as a multicentre study. One example is the EPOCH study that was carried out at 93 British National Health Service hospitals as a stepped-wedge cluster-randomized trial, which introduced a 37-point quality-improvement protocol for 7383 patients and compared the results with those for 8490 patients in a usual-care group32. The postoperative 90-day mortality rate was the same in both of the groups. In a study by Stephens et al.33, which performed a process evaluation of the implementation of the EPOCH study, the results show that 35 per cent of the hospitals were following the 37 points closely and, in more than half, only 11 out of 37 points were implemented. Such a solution would probably help to reduce the length of the SMASH study, but with the risk of compromised adherence to the protocol.
The present study also falls short of identifying any significant improvement for the youngest age groups and the presumptive reason may be that the study was underpowered regarding the outcomes for these age groups. Furthermore, no assessment of clinical frailty has been carried out34, which is obviously a limitation, as the study presents the results for a seriously ill subgroup of elderly surgical patients. However, during the study design in 2017, an evaluation of clinical frailty was not a part of everyday clinical practice and it was therefore not included as a variable in the present study. Today, the use of the clinical frailty scale is recommended for emergency laparotomy29. Finally, in the SMASH study, the patients who had an indication to undergo an emergency laparotomy but who, for various reasons, were not operated on are not registered. This group is categorized as the No-LAP population. Only a few studies regarding the No-LAP population exist and in the future it will be important to include it in cohorts when analysing outcomes after emergency laparotomy. A prospective Scottish study by McIlveen et al.35 shows that No-LAP patients can account for as much as 32 per cent of a total cohort (100 of 314) and a 30-day mortality rate of 63 per cent is reported. Furthermore, in a prospective Danish study from 2023, Ebrahim et al.36 report a lower proportion of No-LAP patients (8.3 per cent of the total cohort of 252 patients), but a 30-day mortality rate of 95 per cent is reported for the No-LAP patients. Consequently, the No-LAP population is still unexplored and undefined and could possibly affect total postoperative mortality rates. It is a limitation of the SMASH study that the No-LAP population is not considered.
The surgical centre at NÄL County Hospital manages all acute surgical patients in its catchment area of 300 000 people. As a result, the cohort of 1344 patients appears to be representative of an unselected group in a Swedish context and the number of included subjects is sufficient for the present study design. However, it would be of scientific interest to conduct an extended study in the form of a multicentre study, with the opportunity to include a larger cohort, providing the opportunity to explore outcomes in a specified subgroup.
The results of the SMASH study, including those presented here, together with previous literature in the field of high-risk emergency abdominal surgery, establish the fact that standardized management protocols produce improved outcomes7,10,12,16. As a result, the implementation of context-adapted standardized management protocols in healthcare systems should be a priority. To improve the opportunities for follow-up still further, the introduction of a Swedish national quality audit would be of great value.
Supplementary Material
Acknowledgements
The authors acknowledge Professor Juri Kartus for overall support, Jeanette Kliger for linguistic assistance, and Per Ekman (Statistiska Konsultgruppen) for data analysis.
Contributor Information
Terje Jansson Timan, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg, Sweden; Department of Research and Development, NU Hospital Group, Trollhättan, Sweden; Department of Anaesthesiology and Intensive Care, NU Hospital Group, Trollhättan, Sweden.
Niklas Ekerstad, Department of Research and Development, NU Hospital Group, Trollhättan, Sweden; Department of Health, Medicine, and Caring Sciences, Unit of Health Care Analysis, Linköping University, Linköping, Sweden.
Ove Karlsson, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg, Sweden.
Ninni Sernert, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg, Sweden; Department of Research and Development, NU Hospital Group, Trollhättan, Sweden.
Mattias Prytz, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg, Sweden; Department of Research and Development, NU Hospital Group, Trollhättan, Sweden; Department of Surgery, NU Hospital Group, Trollhättan, Sweden.
Funding
This work was supported by the Department of Research and Development, NU Hospital Group. Grants were received from the Local Research and Development Council Fyrbodal (VGFOUFBD-803271) and the W&M Lundgren Science Foundation (2019-2883). The two funding parties had no impact on the study design, data collection, analysis, or interpretation of the results in the study.
Author contributions
Terje Jansson Timan (Formal analysis, Writing—original draft), Niklas Ekerstad (Writing—review & editing), Ove Karlsson (Writing—review & editing), Ninni Sernert (Writing—review & editing), and Mattias Prytz (Supervision, Formal analysis, Writing—review & editing).
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data sets analysed during the present study and statistical code are available from the corresponding author on reasonable request.
References
- 1. Scott JW, Olufajo OA, Brat GA, Rose JA, Zogg CK, Haider AH et al. Use of national burden to define operative emergency general surgery. JAMA Surg 2016;151:e160480. [DOI] [PubMed] [Google Scholar]
- 2. Havens JM, Peetz AB, Do WS, Cooper Z, Kelly E, Askari R et al. The excess morbidity and mortality of emergency general surgery. J Trauma Acute Care Surg 2015;78:306–311 [DOI] [PubMed] [Google Scholar]
- 3. Liljendahl MS, Gögenur I, Thygesen LC. Emergency laparotomy in Denmark: a nationwide descriptive study. World J Surg 2020;44:2976–2981 [DOI] [PubMed] [Google Scholar]
- 4. Nally DM, Sørensen J, Valentelyte G, Hammond L, McNamara D, Kavanagh DO et al. Volume and in-hospital mortality after emergency abdominal surgery: a national population-based study. BMJ Open 2019;9:e032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saunders DI, Murray D, Pichel AC, Varley S, Peden CJ. Variations in mortality after emergency laparotomy: the first report of the UK emergency laparotomy network. Br J Anaesth 2012;109:368–375 [DOI] [PubMed] [Google Scholar]
- 6. Tolstrup MB, Watt SK, Gögenur I. Morbidity and mortality rates after emergency abdominal surgery: an analysis of 4346 patients scheduled for emergency laparotomy or laparoscopy. Langenbecks Arch Surg 2017;402:615–623 [DOI] [PubMed] [Google Scholar]
- 7. Aggarwal G, Peden CJ, Mohammed MA, Pullyblank A, Williams B, Stephens T et al. Evaluation of the collaborative use of an evidence-based care bundle in emergency laparotomy. JAMA Surg 2019;154:e190145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Temimi MH, Griffee M, Enniss TM, Preston R, Vargo D, Overton S et al. When is death inevitable after emergency laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement Program database. J Am Coll Surg 2012;215:503–511 [DOI] [PubMed] [Google Scholar]
- 9. Barazanchi AWH, Xia W, MacFater W, Bhat S, MacFater H, Taneja A et al. Risk factors for mortality after emergency laparotomy: scoping systematic review. ANZ J Surg 2020;90:1895–1902 [DOI] [PubMed] [Google Scholar]
- 10. Huddart S, Peden CJ, Swart M, McCormick B, Dickinson M, Mohammed MA et al. Use of a pathway quality improvement care bundle to reduce mortality after emergency laparotomy. Br J Surg 2015;102:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansson Timan T, Hagberg G, Sernert N, Karlsson O, Prytz M. Mortality following emergency laparotomy: a Swedish cohort study. BMC Surg 2021;21:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tengberg LT, Bay-Nielsen M, Bisgaard T, Cihoric M, Lauritsen ML, Foss NB. Multidisciplinary perioperative protocol in patients undergoing acute high-risk abdominal surgery. Br J Surg 2017;104:463–471 [DOI] [PubMed] [Google Scholar]
- 13. Vester-Andersen M, Lundstrom LH, Moller MH, Waldau T, Rosenberg J, Moller AM. Mortality and postoperative care pathways after emergency gastrointestinal surgery in 2904 patients: a population-based cohort study. Br J Anaesth 2014;112:860–870 [DOI] [PubMed] [Google Scholar]
- 14. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–377 [DOI] [PubMed] [Google Scholar]
- 15. Peden C, Scott MJ. Anesthesia for emergency abdominal surgery. Anesthesiol Clin 2015;33:209–221 [DOI] [PubMed] [Google Scholar]
- 16. Timan TJ, Karlsson O, Sernert N, Prytz M. Standardized perioperative management in acute abdominal surgery: Swedish SMASH controlled study. Br J Surg 2023;110:710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watt DG, Wilson MS, Shapter OC Patil P. 30-day and 1-year mortality in emergency general surgery laparotomies: an area of concern and need for improvement? Eur J Trauma Emerg Surg 2015;41:369–374 [DOI] [PubMed] [Google Scholar]
- 18. Jeppesen MM, Thygesen LC, Ekeloef S, Gögenur I. A nationwide cohort study of short- and long-term outcomes following emergency laparotomy. Dan Med J 2019;66:A5523. [PubMed] [Google Scholar]
- 19. Fagan G, Barazanchi A, Coulter G, Leeman M, Hill AG, Eglinton TW. New Zealand and Australia emergency laparotomy mortality rates compare favourably to international outcomes: a systematic review. ANZ J Surg 2021;91:2583–2591 [DOI] [PubMed] [Google Scholar]
- 20. Lee KC, Streid J, Sturgeon D, Lipsitz S, Weissman JS, Rosenthal RA et al. The impact of frailty on long-term patient-oriented outcomes after emergency general surgery: a retrospective cohort study. J Am Geriatr Soc 2020;68:1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng ZQ, Weber D. One-year outcomes following emergency laparotomy: a systematic review. World J Surg 2022;46:512–523 [DOI] [PubMed] [Google Scholar]
- 22. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 23. Timan TJ, Sernert N, Karlsson O, Prytz M. SMASH standardised perioperative management of patients operated with acute abdominal surgery in a high-risk setting. BMC Res Notes 2020;13:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lipsitz SR, Kim K, Zhao L. Analysis of repeated categorical data using generalized estimating equations. Stat Med 1994;13:1149–1163 [DOI] [PubMed] [Google Scholar]
- 25. Aitken RM, Partridge JSL, Oliver CM, Murray D, Hare S, Lockwood S et al. Older patients undergoing emergency laparotomy: observations from the National Emergency Laparotomy Audit (NELA) years 1–4. Age Ageing 2020;49:656–663 [DOI] [PubMed] [Google Scholar]
- 26. Peacock O, Bassett MG, Kuryba A, Walker K, Davies E, Anderson I et al. Thirty-day mortality in patients undergoing laparotomy for small bowel obstruction. Br J Surg 2018;105:1006–1013 [DOI] [PubMed] [Google Scholar]
- 27. Ylimartimo AT, Lahtinen S, Nurkkala J, Koskela M, Kaakinen T, Vakkala M et al. Long-term outcomes after emergency laparotomy: a retrospective study. J Gastrointest Surg 2022;26:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliver CM, Bassett MG, Poulton TE, Anderson ID, Murray DM, Grocott MP et al. Organisational factors and mortality after an emergency laparotomy: multilevel analysis of 39 903 National Emergency Laparotomy Audit patients. Br J Anaesth 2018;121:1346–1356 [DOI] [PubMed] [Google Scholar]
- 29. Peden CJ, Aggarwal G, Aitken RJ, Anderson ID, Bang Foss N, Cooper Z et al. Guidelines for perioperative care for emergency laparotomy Enhanced Recovery After Surgery (ERAS) Society recommendations: part 1—preoperative: diagnosis, rapid assessment and optimization. World J Surg 2021;45:1272–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khadaroo RG, Warkentin LM, Wagg AS, Padwal RS, Clement F, Wang X et al. Clinical effectiveness of the Elder-Friendly Approaches to the Surgical Environment initiative in emergency general surgery. JAMA Surg 2020;155:e196021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Girard E, Abba J, Boussat B, Trilling B, Mancini A, Bouzat P et al. Damage control surgery for non-traumatic abdominal emergencies. World J Surg 2018;42:965–973 [DOI] [PubMed] [Google Scholar]
- 32. Peden CJ, Stephens T, Martin G, Kahan BC, Thomson A, Rivett K et al. Effectiveness of a national quality improvement programme to improve survival after emergency abdominal surgery (EPOCH): a stepped-wedge cluster-randomised trial. Lancet 2019;393:2213–2221 [DOI] [PubMed] [Google Scholar]
- 33. Stephens TJ, Peden CJ, Pearse RM, Shaw SE, Abbott TEF, Jones EL et al. Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (EPOCH trial). Implement Sci 2018;13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr 2020;20:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McIlveen EC, Wright E, Shaw M, Edwards J, Vella M, Quasim T et al. A prospective cohort study characterising patients declined emergency laparotomy: survival in the ‘NoLap’ population. Anaesthesia 2020;75:54–62 [DOI] [PubMed] [Google Scholar]
- 36. Ebrahim M, Lauritsen ML, Cihoric M, Hilsted KL, Foss NB. Triage and outcomes for a whole cohort of patients presenting for major emergency abdominal surgery including the No-LAP population: a prospective single-center observational study. Eur J Trauma Emerg Surg 2023;49:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analysed during the present study and statistical code are available from the corresponding author on reasonable request.
References
- 1. Scott JW, Olufajo OA, Brat GA, Rose JA, Zogg CK, Haider AH et al. Use of national burden to define operative emergency general surgery. JAMA Surg 2016;151:e160480. [DOI] [PubMed] [Google Scholar]
- 2. Havens JM, Peetz AB, Do WS, Cooper Z, Kelly E, Askari R et al. The excess morbidity and mortality of emergency general surgery. J Trauma Acute Care Surg 2015;78:306–311 [DOI] [PubMed] [Google Scholar]
- 3. Liljendahl MS, Gögenur I, Thygesen LC. Emergency laparotomy in Denmark: a nationwide descriptive study. World J Surg 2020;44:2976–2981 [DOI] [PubMed] [Google Scholar]
- 4. Nally DM, Sørensen J, Valentelyte G, Hammond L, McNamara D, Kavanagh DO et al. Volume and in-hospital mortality after emergency abdominal surgery: a national population-based study. BMJ Open 2019;9:e032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saunders DI, Murray D, Pichel AC, Varley S, Peden CJ. Variations in mortality after emergency laparotomy: the first report of the UK emergency laparotomy network. Br J Anaesth 2012;109:368–375 [DOI] [PubMed] [Google Scholar]
- 6. Tolstrup MB, Watt SK, Gögenur I. Morbidity and mortality rates after emergency abdominal surgery: an analysis of 4346 patients scheduled for emergency laparotomy or laparoscopy. Langenbecks Arch Surg 2017;402:615–623 [DOI] [PubMed] [Google Scholar]
- 7. Aggarwal G, Peden CJ, Mohammed MA, Pullyblank A, Williams B, Stephens T et al. Evaluation of the collaborative use of an evidence-based care bundle in emergency laparotomy. JAMA Surg 2019;154:e190145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Temimi MH, Griffee M, Enniss TM, Preston R, Vargo D, Overton S et al. When is death inevitable after emergency laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement Program database. J Am Coll Surg 2012;215:503–511 [DOI] [PubMed] [Google Scholar]
- 9. Barazanchi AWH, Xia W, MacFater W, Bhat S, MacFater H, Taneja A et al. Risk factors for mortality after emergency laparotomy: scoping systematic review. ANZ J Surg 2020;90:1895–1902 [DOI] [PubMed] [Google Scholar]
- 10. Huddart S, Peden CJ, Swart M, McCormick B, Dickinson M, Mohammed MA et al. Use of a pathway quality improvement care bundle to reduce mortality after emergency laparotomy. Br J Surg 2015;102:57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansson Timan T, Hagberg G, Sernert N, Karlsson O, Prytz M. Mortality following emergency laparotomy: a Swedish cohort study. BMC Surg 2021;21:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tengberg LT, Bay-Nielsen M, Bisgaard T, Cihoric M, Lauritsen ML, Foss NB. Multidisciplinary perioperative protocol in patients undergoing acute high-risk abdominal surgery. Br J Surg 2017;104:463–471 [DOI] [PubMed] [Google Scholar]
- 13. Vester-Andersen M, Lundstrom LH, Moller MH, Waldau T, Rosenberg J, Moller AM. Mortality and postoperative care pathways after emergency gastrointestinal surgery in 2904 patients: a population-based cohort study. Br J Anaesth 2014;112:860–870 [DOI] [PubMed] [Google Scholar]
- 14. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–377 [DOI] [PubMed] [Google Scholar]
- 15. Peden C, Scott MJ. Anesthesia for emergency abdominal surgery. Anesthesiol Clin 2015;33:209–221 [DOI] [PubMed] [Google Scholar]
- 16. Timan TJ, Karlsson O, Sernert N, Prytz M. Standardized perioperative management in acute abdominal surgery: Swedish SMASH controlled study. Br J Surg 2023;110:710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watt DG, Wilson MS, Shapter OC Patil P. 30-day and 1-year mortality in emergency general surgery laparotomies: an area of concern and need for improvement? Eur J Trauma Emerg Surg 2015;41:369–374 [DOI] [PubMed] [Google Scholar]
- 18. Jeppesen MM, Thygesen LC, Ekeloef S, Gögenur I. A nationwide cohort study of short- and long-term outcomes following emergency laparotomy. Dan Med J 2019;66:A5523. [PubMed] [Google Scholar]
- 19. Fagan G, Barazanchi A, Coulter G, Leeman M, Hill AG, Eglinton TW. New Zealand and Australia emergency laparotomy mortality rates compare favourably to international outcomes: a systematic review. ANZ J Surg 2021;91:2583–2591 [DOI] [PubMed] [Google Scholar]
- 20. Lee KC, Streid J, Sturgeon D, Lipsitz S, Weissman JS, Rosenthal RA et al. The impact of frailty on long-term patient-oriented outcomes after emergency general surgery: a retrospective cohort study. J Am Geriatr Soc 2020;68:1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ng ZQ, Weber D. One-year outcomes following emergency laparotomy: a systematic review. World J Surg 2022;46:512–523 [DOI] [PubMed] [Google Scholar]
- 22. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 23. Timan TJ, Sernert N, Karlsson O, Prytz M. SMASH standardised perioperative management of patients operated with acute abdominal surgery in a high-risk setting. BMC Res Notes 2020;13:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lipsitz SR, Kim K, Zhao L. Analysis of repeated categorical data using generalized estimating equations. Stat Med 1994;13:1149–1163 [DOI] [PubMed] [Google Scholar]
- 25. Aitken RM, Partridge JSL, Oliver CM, Murray D, Hare S, Lockwood S et al. Older patients undergoing emergency laparotomy: observations from the National Emergency Laparotomy Audit (NELA) years 1–4. Age Ageing 2020;49:656–663 [DOI] [PubMed] [Google Scholar]
- 26. Peacock O, Bassett MG, Kuryba A, Walker K, Davies E, Anderson I et al. Thirty-day mortality in patients undergoing laparotomy for small bowel obstruction. Br J Surg 2018;105:1006–1013 [DOI] [PubMed] [Google Scholar]
- 27. Ylimartimo AT, Lahtinen S, Nurkkala J, Koskela M, Kaakinen T, Vakkala M et al. Long-term outcomes after emergency laparotomy: a retrospective study. J Gastrointest Surg 2022;26:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliver CM, Bassett MG, Poulton TE, Anderson ID, Murray DM, Grocott MP et al. Organisational factors and mortality after an emergency laparotomy: multilevel analysis of 39 903 National Emergency Laparotomy Audit patients. Br J Anaesth 2018;121:1346–1356 [DOI] [PubMed] [Google Scholar]
- 29. Peden CJ, Aggarwal G, Aitken RJ, Anderson ID, Bang Foss N, Cooper Z et al. Guidelines for perioperative care for emergency laparotomy Enhanced Recovery After Surgery (ERAS) Society recommendations: part 1—preoperative: diagnosis, rapid assessment and optimization. World J Surg 2021;45:1272–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khadaroo RG, Warkentin LM, Wagg AS, Padwal RS, Clement F, Wang X et al. Clinical effectiveness of the Elder-Friendly Approaches to the Surgical Environment initiative in emergency general surgery. JAMA Surg 2020;155:e196021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Girard E, Abba J, Boussat B, Trilling B, Mancini A, Bouzat P et al. Damage control surgery for non-traumatic abdominal emergencies. World J Surg 2018;42:965–973 [DOI] [PubMed] [Google Scholar]
- 32. Peden CJ, Stephens T, Martin G, Kahan BC, Thomson A, Rivett K et al. Effectiveness of a national quality improvement programme to improve survival after emergency abdominal surgery (EPOCH): a stepped-wedge cluster-randomised trial. Lancet 2019;393:2213–2221 [DOI] [PubMed] [Google Scholar]
- 33. Stephens TJ, Peden CJ, Pearse RM, Shaw SE, Abbott TEF, Jones EL et al. Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (EPOCH trial). Implement Sci 2018;13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr 2020;20:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McIlveen EC, Wright E, Shaw M, Edwards J, Vella M, Quasim T et al. A prospective cohort study characterising patients declined emergency laparotomy: survival in the ‘NoLap’ population. Anaesthesia 2020;75:54–62 [DOI] [PubMed] [Google Scholar]
- 36. Ebrahim M, Lauritsen ML, Cihoric M, Hilsted KL, Foss NB. Triage and outcomes for a whole cohort of patients presenting for major emergency abdominal surgery including the No-LAP population: a prospective single-center observational study. Eur J Trauma Emerg Surg 2023;49:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]