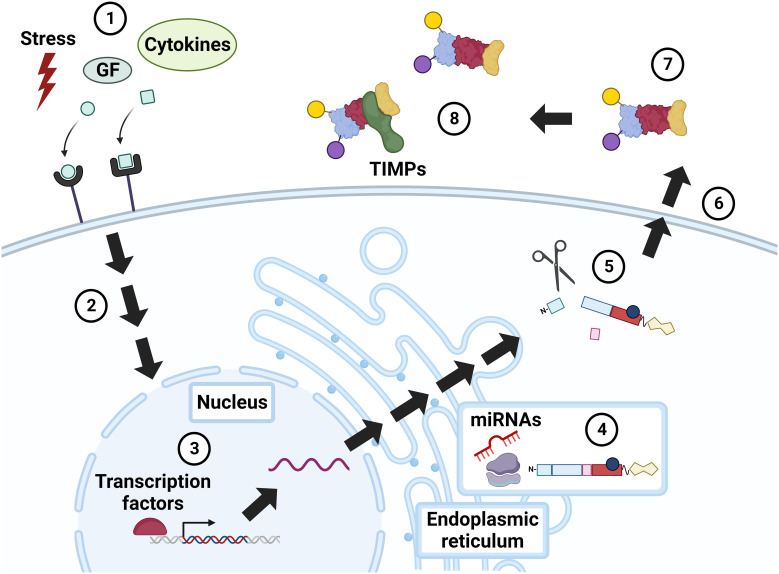
Figure 2.
Regulation of MMP activity. Constitutive MMP expression is augmented in response to external stimuli, e.g., physiochemical stress, growth factors (GF), and cytokines (1), that incite intracellular signaling via mitogen activated protein kinase (MAPK), NF-kB, Smad, and others (2). MMP expression is further modulated by transcription factors, notably AP-1 (3). MMP RNA is translated into a pre-propeptide (4), a process that can be up- and down-regulated by microRNAs (miRNAs). Cleavage of the N-terminal signal peptide in the endoplasmic reticulum (ER) yields the pro-MMP zymogen, which may undergo intracellular (e.g., cleavage by furin) and extracellular (e.g., cleavage by plasmin) modification (5). Cellular release of MMPs is also regulated by stress, growth factors, and cytokines (6). Extracellular MMPs are activated by post-translational modifications (7), or by the action of other, membrane-bound MMPs. MMP activity is also modulated by complexes formed with inhibitors, i.e., TIMPs and α2-macroglobulin (8). GF, growth factors; TIMP, tissue inhibitor of metalloproteinase. Created with BioRender.com.