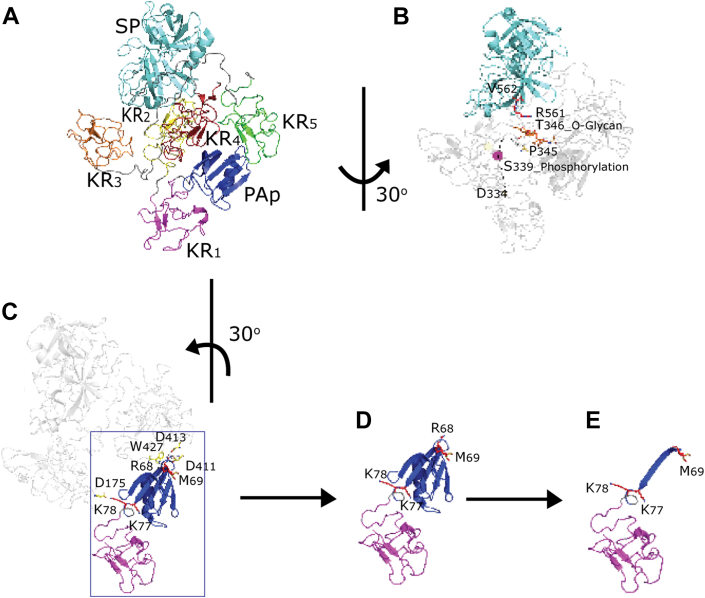
Fig. 6.
Plasminogen (Plg) phosphorylation and activation.A and B, X-ray crystal structure of human Plg (Protein Data Bank ID: 4DUR) in the closed conformation. Plg, a seven-domain protein, from the N terminus, Pan-apple domain (PAp, blue), kringles 1 to 5 domain (KR1–5, magenta, yellow, orange, red, and green, respectively), and the serine protease domain (SP, cyan). B, the phosphorylated S339 is located at the loop (residues 334–356) connecting KR3 and KR4. In the crystal structure, residues 335 to 344 cannot be accurately modeled based on the electronic map suggesting this region is flexible. Here, it is represented by a dotted line with the phosphorylated S339 modeled. In the closed conformation, this loop is near the activation loop R561|V562 (red sticks). C, in the closed conformation, in the first cleavage site R68|M69 (red sticks), the P1 residue R68 binds to KR4 via the main chain with W427 (yellow sticks) and the side chain with D411 and D413 (yellow sticks) from the lysine-binding site. In the second cleavage site K77|K78 (red sticks), the P1 residue K77 is buried within the PAp domain, and the P1 residue K78 bonds with D175 of KR2 (yellow sticks). As a result, when in the closed position, both sites are predicted to be inaccessible. D, in the open conformation, the first cleavage site becomes accessible, whilst the second cleavage site remains restricted by the PAp domain. E, upon cleavage of the first site, the second cleavage site becomes exposed.