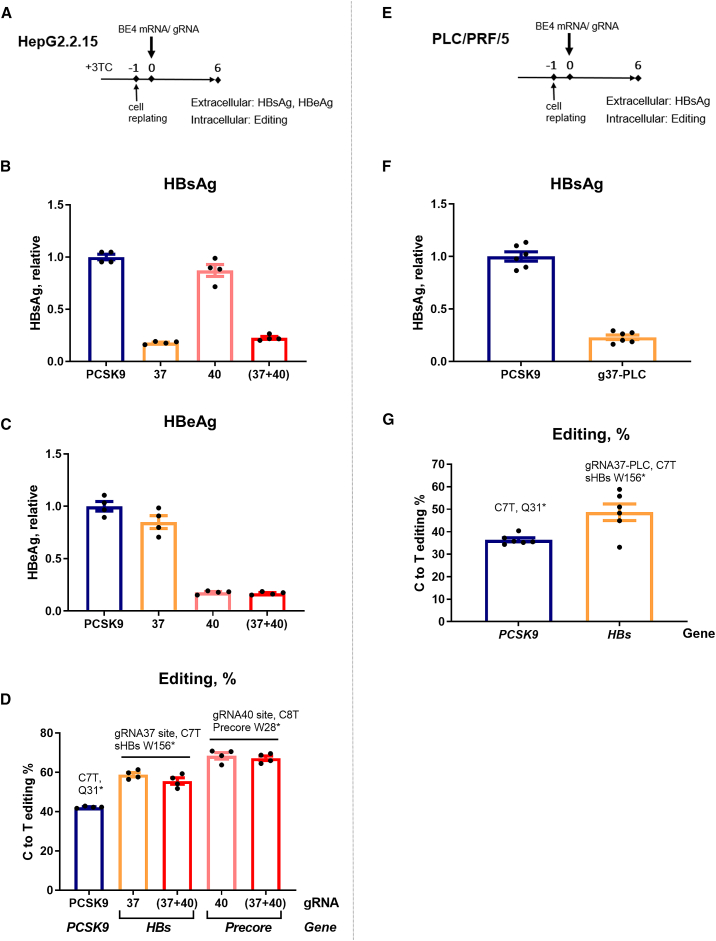
Figure 4.
Anti-HBV efficacy of CBE and gRNAs in HBV-integrated cell lines
(A) Schematic representation of the protocol used for HepG2.2.15 cells. All of the samples were collected 6 days posttransfection (dpt). (B and C) Extracellular HBsAg and HBeAg levels were assessed in the supernatant of the cells treated with 3TC 4 days before the transfection with base editing reagents. (D) Level of the C>T functional editing was assessed by NGS on the purified DNA. (E) Schematic representation of the protocol used for PLC/PRF/5 cells. (F) At 6 dpt, extracellular HBsAg was measured in the supernatants of the cells transfected with g37-PLC (g37 adapted for genotype A HBs targeting site within PLC/PLF/5 cells). (G) The level of the C>T editing on the HBs targeting site of g37-PLC was assessed by NGS. All of the ELISA data were normalized to the BE4/PCSK9 (control gRNA) condition. Error bars indicate SEM of 4 or 6 replicates.