Abstract
Introduction
The population-based Inter99 cohort has contributed extensively to our understanding of effects of a systematic screening and lifestyle intervention, as well as the multifactorial aetiology of type 2 diabetes (T2D) and cardiovascular disease. To understand causes, trajectories and patterns of early and overt cardiometabolic disease manifestations, we will perform a combined clinical deep phenotyping and registry follow-up study of the now 50–80 years old Inter99 participants.
Methods and analysis
The Inter99 cohort comprises individuals aged 30–60 years, who lived in a representative geographical area of greater Copenhagen, Denmark, in 1999. Age-stratified and sex-stratified random subgroups were invited to participate in either a lifestyle intervention (N=13 016) or questionnaires (N=5264), while the rest served as a reference population (N=43 021). Of the 13 016 individuals assigned to the lifestyle intervention group, 6784 (52%) accepted participation in a baseline health examination in 1999, including screening for cardiovascular risk factors and prediabetic conditions. In total, 6004 eligible participants, who participated in the baseline examination, will be invited to participate in the deep phenotyping 20-year follow-up clinical examination including measurements of anthropometry, blood pressure, arterial stiffness, cardiometabolic biomarkers, coronary artery calcification, heart rate variability, heart rhythm, liver stiffness, fundus characteristics, muscle strength and mass, as well as health and lifestyle questionnaires. In a subsample, 10-day monitoring of diet, physical activity and continuous glucose measurements will be performed. Fasting blood, urine and faecal samples to be stored in a biobank. The established database will form the basis of multiple analyses. A main purpose is to investigate whether low birth weight independent of genetics, lifestyle and glucose tolerance predicts later common T2D cardiometabolic comorbidities.
Ethics and dissemination
The study was approved by the Medical Ethics Committee, Capital Region, Denmark (H-20076231) and by the Danish Data Protection Agency through the Capital Region of Denmark’s registration system (P-2020-1074). Informed consent will be obtained before examinations. Findings will be disseminated in peer-reviewed journals, at conferences and via presentations to stakeholders, including patients and public health policymakers.
Trial registration number
Keywords: DIABETES & ENDOCRINOLOGY, CARDIOLOGY, GENETICS, NUTRITION & DIETETICS, EPIDEMIOLOGY, REGISTRIES
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The longitudinal design will enable us to follow the course of both early and overt cardiometabolic disease manifestations during the period of life with highest incidence rates.
We will be able to quantify the extent to which the type 2 diabetes (T2D)-associated comorbidities coronary arteriosclerosis, cardiac autonomic neuropathy, non-alcoholic fatty liver disease, retinopathy and diabetic kidney disease are present among elderly people without T2D, and with known normal glucose tolerance for two decades.
The availability of genome-wide genetic variation data, birth weight, as well as adiposity trajectories, dietary data, and physical activity information, will provide insights into how these predisposing factors influence distinct organ morbidities and disease manifestations.
Collection of biospecimens for micronutrient and multiomics purposes such as genomics, transcriptomics, proteomics, metabolomics, lipidomics and epigenomics will allow additional layers of deep phenotype analyses, including studying disease-associated genetic variants and their phenotypes.
The fact that all individuals participated in a screening for cardiovascular disease risk and, if at risk, invited for a personalised lifestyle intervention from 1999 and for up to 5 years thereafter, may limit the generalisability of our findings.
Introduction
Type 2 diabetes (T2D), cardiovascular disease (CVD) and their comorbidities are leading causes of premature mortality and morbidity, affecting nearly one billion individuals worldwide.1 2 T2D is arbitrarily defined by elevations of plasma glucose levels, and the current T2D diagnostic criteria do not capture the diversity of T2D subphenotypes characterised by differential manifestations of microvascular and macrovascular complications, as well as other common comorbidities.3 4
The overlap and heterogeneity of age-related T2D, CVD and associated comorbidities are likely rooted in the relative or predominant contributions from the triad of genetic susceptibility versus prenatal and postnatal non-genetic aetiologies (box 1).
Box 1. The complex multifactorial prenatal and postnatal aetiology of cardiometabolic diseases.
Constitutional primary predisposing factors
Genetic susceptibility.
Intrauterine environment (low or high birth weight, prematurity).
Acquired postnatal secondary precipitating factors
Sedentary lifestyle/inactivity.
Unhealthy diet/micronutrient deficiencies.
Obesity.
Smoking.
Medication.
Comorbidities.
Ageing.
As for genetics, the known 568 T2D susceptibility loci are estimated to explain 18% of the putative genetic contribution to T2D.5 6 Early life developmental programming, low birth weight (LBW), as well as salt-sensitive hypertension, non-alcoholic fatty acid disease (NAFLD), dyslipidaemia and neurocognitive dysfunctions, are well-established risk factors of T2D and CVD.7–12 Recent data even suggest that LBW, in a non-genetic manner, is associated with a more severe clinical T2D presentation and course, including earlier onset and more comorbidities at the time of diagnosis.13 Accordingly, there is an increasing need to understand whether differential combinations of T2D aetiologies influence not only T2D and CVD risk per se, but also the timing and patterns of clinical presentation including both early and late disease manifestations, as well as comorbidities. As an example of unprioritised comorbidities, patients with T2D have a 2–3 fold increased risk of sarcopenia.14 15 Sarcopenia describes the age-related loss of muscle mass and strength that leads to impaired function including increased risk of falls and an overall decreased quality of life. Sarcopenia is accelerated by physical inactivity, low protein intake, and general health status and disease, and has also been associated with LBW.16
Excess dietary sodium (Na+) may account for three million deaths annually,17 and reducing salt intake is among the most cost-effective CVD prevention strategies.18 Low dietary potassium (K+) intake is also gaining attention as a CVD risk factor, and the urinary Na+/K+-ratio may, therefore, represent a superior cardiovascular risk measure.19–23 As for macronutrient intake, high dietary sugar and saturated fat contents is strongly associated with T2D and CVD risk. While the Mediterranean diet may prevent CVD,24 there is nevertheless substantial gaps in our current knowledge of what defines a healthy diet with respect to not only macronutritional but also micronutritional composition(s) including vitamins. For instance, beyond effects on blood clotting factors, vitamin K may be important for cardiometabolic as well as bone health.25
The Inter99 cohort provides a unique research platform to delineate the differential and overlapping roles of genetics versus the fetal environmental, as well as various postnatal lifestyle factors, for the development of early and overt cardiometabolic disease manifestations and associated comorbidities.26–28 We aim to perform a combined deep phenotyping and registry-based follow-up study of the Inter99 cohort, 20 years after the baseline health examinations, when participants were on average 46 years of age. While overt disease diagnoses will be captured by Danish national registries, our deep phenotyping clinical examinations allow detection of a wider range of early disease manifestations, such as vascular stiffness, liver fibrosis, retinopathy, diabetic kidney disease and pre-diabetes, and the extent to which these may be present prior to the participants complying with official cardiometabolic disease diagnoses.
Methods and analysis
Study setting and previous findings
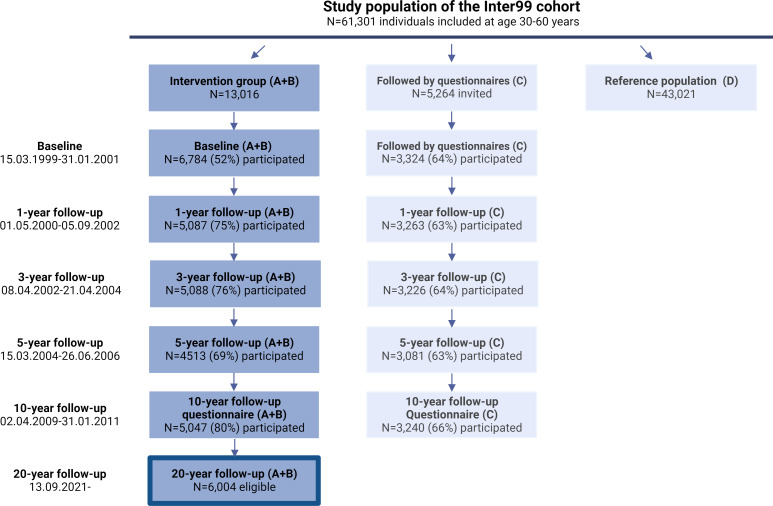
The Inter99 study was initiated in March 1999 as a population-based multifactorial intervention study, originally designed to prevent ischaemic heart disease (IHD).29 It comprised all individuals born in 1939–1940, 1944–1945, 1949–1950, 1954–1955, 1959–1960, 1964–1965 and 1969–1970 (30, 35, 40, 45, 50, 55 and 60 years of age) living in 11 municipalities in Greater Copenhagen (N=61 301). The population was randomised with different age and sex ratios to two lifestyle intervention groups (A+B; N=13 016) or a group followed by questionnaires (C; N=5264), the remaining individuals were considered as a reference population and not contacted (D; N=43 021) (figure 1). Participants received individualised lifestyle counselling based on lifestyle and CVD risk score.30 In total, 6784 participants from the intervention groups participated in the baseline health examination (52% acceptance rate).29 The 5-year clinical follow-up examination, including glucose tolerance status, had a participation rate of 69%.31 The 10-year follow-up was based on registry data and a follow-up questionnaire was sent to all eligible participants with completed baseline health examination (A+B) and to all in the questionnaire group (C) (figure 1).32
Figure 1.
Flow chart of participation in the Inter99 study, 1999–2023. The dark blue column represents the Intervention group (A+B) where 52% of the invited had a baseline examination performed in 1999. The eligible participants for the 20-year follow-up study are recruited among these individuals. The light-blue columns indicate individuals followed by questionnaires (C) and the reference population (D) who are not recruited for the 20-year follow-up examinations.
The 5-year follow-up examination showed a progression rate to overt T2D of 2.1 per 100 person-years,33 strongly supported by a substantially higher T2D prevalence after 20 years of follow-up with 6.5% of men and 3.8% in women having been diagnosed with T2D. Indeed, this will also be the case for all other T2D-associated vascular and cardiometabolic comorbidities, underscoring the relevance of performing a combined registry-based and cardiometabolic deep phenotyping study among Inter99 participants, who are now 50–80 years old.
Original midwife records were collected from 4744 participants in the intervention groups,34 35 and despite the relatively low average age of 46 years in 1999, we confirmed a strong inverse relationship between birth weight and risk of T2D in this Danish population.35 The Inter99 cohort has been extensively genotyped contributing to the identification of more than 568 T2D susceptibility loci,5 and to the interactions between birth weight and genetic risk of T2D.27 28 36 The prevalence of T2D associated retinopathy in the Inter99 cohort was studied in a subgroup of 970 participants. Interestingly, retinopathy was present in 7.5% of the 490 subjects with completely normal glucose tolerance, supporting the notion that factors other than elevated glucose contribute to the risk of retinopathy of a type that cannot be distinguished from milder degrees of diabetic retinopathy.37
The 10-year follow-up study concluded that a community-based, individually tailored intervention programme with screening for risk of IHD and repeated lifestyle intervention over 5 years, had no effect on IHD, stroke or mortality at the population level as assessed after 10 years.38 This observation was later reproduced in other studies and confirmed in a WHO report.39
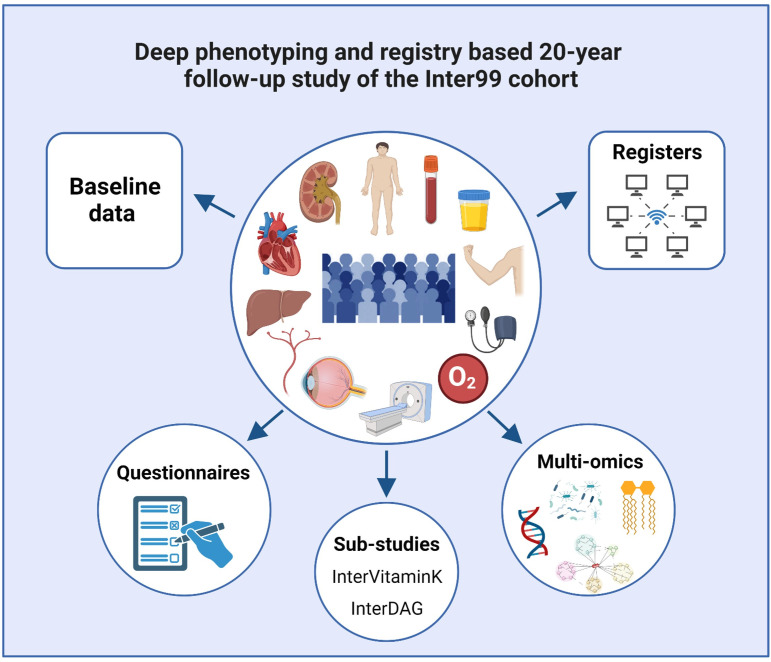
The totality of data already available in the Inter99 cohort range from information on birth size (weight and length) and prematurity, glucose tolerance at baseline and at a 5-year follow-up, lifestyle intervention and general health information including comprehensive dietary data, numerous biomarkers and genetic data (table 1, figure 2). The 20-year follow-up study includes physical deep phenotyping clinical examinations tailored to capture and expand our growing understanding of T2D and subgroups, as well as early and late metabolic and vascular manifestations, comorbidities and complications. Morbidity and mortality data are obtained from our extensive Danish registers. The existing detailed clinical and lifestyle information over 20 years will allow us to correct for those determinants in the analysis. This together will allow us to detect effects of age-related cardiovascular and metabolic phenotypes.
Table 1.
Summary of data collected at the Inter99 baseline and the 20-year follow-up study (including extended substudies)
| Data | Variables | Baseline | 20 years |
| Questionnaire-based information | |||
| Demographics | Sex, age, family, marital status, education, employment status, household income | X | X |
| Diseases | Chronic diseases, contact to healthcare system, symptoms | X | X |
| Health | Self-rated health, stress, sleep | X | X |
| Lifestyle | Physical activity, smoking, alcohol, diet, network | X | X |
| Deep-phenotyping health assessment | |||
| Anthropometry | Height, weight, waist and hip circumference | X | X |
| Bioelectrical impedance | Fat and lean (muscle) body mass | X | |
| Blood pressure | Systolic and diastolic blood pressure, resting heart rate | X | X |
| Cardiac autonomic neuropathy | Resting heart rate variability and cardiovascular autonomic reflex tests | X | |
| Cardiac CT | Coronary atherosclerosis, cardiac chamber size, LV hypertrophy | X | |
| Continuous glucose monitoring | 7 day 24-hour glucose levels | X(S) | |
| Dynamometer and sit-to-stand test | Muscle strength | X | |
| Electrocardiography | ECG intervals, amplitudes and diagnostic statements | X | X |
| Ophthalmic examination | Ocular fundus characteristics, retinopathy | X(S) | X |
| Oxymeter | Oxygen saturation | X | |
| Pulse wave velocity | Arterial stiffness | X | |
| Spirometry | Lung function | X | X(S) |
| Transient elastography | Liver stiffness and steatosis | X | |
| Laboratory assessments | |||
| Blood biochemistry | Leucocytes and differential count, thrombocytes, electrolytes (sodium, potassium, calcium), glucose, HbA1c, lipids (total cholesterol, HDL, LDL, VLDL, triglycerides), kidney function (creatinine, eGFR, urea, albumin), vitamin K status (dephosphorylated-uncarboxylated matrix-gla Protein), liver function (ALAT, ASAT) | X | X |
| Urine biochemistry | Albumin, creatinine, sodium, potassium | X | X |
| Biobanking (−80°C) | |||
| Blood | Fasting blood samples (whole-blood, serum and plasma) | X | X |
| Urine | Spot urine 24-hour urine collection |
X X(S) |
X X(S) |
| Faeces | Faecal samples | X(S) | |
ALAT, Alanine transaminase ; ASAT, Aspartate transaminase; eGFR, estimated glomerular filtration rate; HbA1c, Hemoglobin A1c; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; LV, Left ventricular; S, subgroup of participant; VLDL, Very-low-density lipoprotein.
Figure 2.
Overview of the 20-year deep-phenotyping follow-up examinations and possibilities of coupling to baseline and register data as well as for future and extended analyses. DAG, diet, activity and glucose.
Deep phenotyping follow-up study
Recruitment and clinical examinations
A search of the Danish civil registration register (CPR register) in December 2019 showed that 6004 (88.5%) of the Inter99 participants, who had participated in the baseline examination, were alive and had not emigrated; and thus, eligible for inclusion (figure 1). There were no other eligibility criteria for study participation.
All participants will be invited in the same order as examined at baseline to the clinical examination at The Center for Clinical Research and Prevention in Glostrup, Denmark. The first participant was examined on 13 September 2021. The data are collected in a highly standardised manner by trained health professionals. Data include repeated measures of the original health examinations (table 1), studies of subclinical signs of early cardiometabolic changes using innovative technologies, lifestyle questionnaires, extended substudies of Diet, Activity and Glucose (the InterDAG study) and a vitamin K supplementation intervention (InterVitaminK trial) as well as collection of biological samples for a biobank (figure 2) as described below and in detail in online supplemental methods.
bmjopen-2023-078501supp001.pdf (127KB, pdf)
Anthropometry and body composition
Height is measured without shoes to the nearest cm, weight without shoes and overcoat to the nearest kg and body mass index (BMI) calculated (kg/m2). Waist and hip circumferences are measured in cm using a non-stretchable tape and waist-to-hip ratio calculated. Segmental body composition is estimated from multifrequency bioelectrical impedance analysis (InBody770, Biospace, Seoul, South Korea).
Arterial stiffness
Arterial stiffness is assessed by the gold standard method of assessing direct arterial stiffness based on carotid-femoral Pulse Wave Velocity using the SphygmoCor XCEL instrument (AtCor Medical, Sydney, Australia).25
Biobank
Serum, plasma and urine will be collected from all participants. In a subgroup participating in the extended InterDAG study, 24-hour urine samples and a faecal sample will be collected. This biobank allows for future analyses of selected biomarkers and multiomics.
Blood biochemistry
Fasting blood samples (minimum of 6 hours) (table 1) are collected and analysed within 3 hours (online supplemental methods).
Blood pressure
Blood pressure is measured thrice with a Microlife BP A6 PC electronic blood pressure monitor (Microlife, Widnau, Switzerland) and fitting cuff after 5 min rest in sitting position.
Cardiac autonomic neuropathy
As a measure of cardiac autonomic neuropathy, simple bedside tests using resting heart rate variability indices, response in heart rate to standing, slow breathing, and the Valsalva manoeuvre (cardiovascular autonomic reflex tests) is used with a Vagus device (Medicus Engineering, Aarhus, Denmark).40 41
Cardiac CT
All participants are offered a cardiac CT scan at the Department of Cardiology, Rigshospitalet in Copenhagen to determine coronary atherosclerosis, including coronary artery calcification score (CAC score), coronary stenosis, vascular extent and plaque type in addition to cardiac chamber size and left ventricular hypertrophy. The cardiac CT scan includes a non-contrast CT scan and a CT angiography performed using the 320-multidetector scanner (Aquilion One, Toshiba Medical Systems, Japan).42 43 In addition to cardiac risk assessment, the CT scan includes comprehensive imaging of lungs, vascular system, sarcopenia assessment, liver, spleen, abdominal fat and spine.
Covariates
At baseline, participants answered a questionnaire on sex, age, marital status, occupation, education, health (diagnoses of, eg, cancer, diabetes, hypertension, high cholesterol, myocardial infarction, stroke or coeliac disease) and lifestyle (physical activity, smoking, alcohol and dietary habits).29 At follow-up participants answer additional questions on sarcopenia symptoms, sleep, sedentary behaviour and physical activity.44–46
ECG
Ten second 12-lead ECG is digitally recorded at 500 hz for 10 s and analysed using the Marquette 12SL algorithm (V.21, GE Healthcare, Milwaukee, Wisconsin, USA).47
Liver stiffness and steatosis
Non-invasive assessment of liver stiffness by transient elastography is performed after a minimum of 4 hours fasting (FibroScan 530 Touch, Echosens, Baarn, Netherlands). The FIB-4 (Fibrosis-4) score will be calculated.48
Muscle strength (hand grip and chair stand)
Muscular fitness is assessed using standardised protocols of muscle performance in the upper and lower extremity. Hand grip is measured using a Jamar dynamometer (Sammons Preston Rolyan, Chicago, Illinois, USA).49 Lower body muscle performance is measured using the Sit-to-Stand test.50
Oxygen saturation
Oxygen saturation is determined by direct measurement with the Nellcor portable SpO2 pulse oximeter after blood sampling and 0 min rest in the supine position. Measurements are taken in the index finger of the opposite arm of blood sampling (Medtronic, Minneapolis, Minnesota, USA).
Fundus characteristics
Ocular wide-field fundus photography and optical coherence tomography are made in both eyes using the Optos Monaco OCT Ophthalmoscope (Optos, Dunfermline, UK).51 Images and scans are graded for retinopathy according to a modified version of the ‘Proposed International Clinical DR severity scale’52 applying a deep learning algorithm by convolutional neural networks.53
Urine
A spot urine sample is collected and analysed for sodium, potassium, albumin and creatinine concentration.
Extended clinical studies
The study serves as a recruitment platform for the InterVitaminK Randomised Controlled Trial,25 as well as for the extended lifestyle and diurnal plasma glucose profiling InterDAG study as described below and in details in online supplemental methods.
InterVitaminK trial
In total, 450 men and women, who participated in the Inter99 20-year follow-up study with detectable CAC (CAC≥10 Agatston units) assessed by the cardiac CT scan, will be recruited to participate in the double-blinded placebo-controlled randomised intervention trial, the InterVitaminK trial, investigating the effect of Vitamin K supplementation on progression of CAC (table 1, figure 2) as described in more detail elsewhere.25
InterDAG study
The InterDAG study will recruit 1000 consecutive participants from the Inter99 follow-up study with no exclusion criteria. Glucose levels are measured continuously for 10 days using continuous glucose monitoring (CGM) (Dexcom G6 PRO, Hudson, Ohio, USA). Simultaneously, comprehensive data on dietary intakes and physical activity are collected including a 7-day food record (MyFood24, www.myfood24.org) and 10-day physical activity (24-hour Sens Motion accelerometers, www.sens.dk, Copenhagen, Denmark). Participants are also invited for a 3-day repeated 24-hour urine collection and one single faecal collection for later analyses. The simultaneous measurements of daily physical activity and diet will increase our understanding of the extent to which current lifestyle in older people influence, not only glucose regulation and variability, but also cardiometabolic health in general, including ectopic fat deposition, sarcopenia and early vascular dysfunctions. The 24-hour urine collection will provide unique data on the sodium to potassium ratio, while the gut microbiota from faecal samples will provide insights into interaction between microbiota and metabolic diseases.54–56
Expected timeline
The cardiometabolic deep-phenotyping study including cardiac CT scans and 7-day continuous monitoring of the InterDAG in a subgroup will be completed in April 2024. Data cleaning, validation and organisation of the database are expected to be completed by end 2024.
Register-based follow-up of the Inter99 cohort
The Inter99 cohort is linked to the nationwide Danish registries by the unique Danish CPR number. The registries cover hospital admissions, outpatient contacts, primary healthcare use, reimbursement of medicine and a variety of social parameters (education, income, employment, ethnicity, etc). Information on date of T2D diagnosis is obtained from a newly established Danish Diabetes Register based on comprehensive data from the National Patient Register,57 the Medicines Products Register,58 the National Health Service Registry,59 the Danish Adult Diabetes Database60 and the Eye Examination Database.61 The algorithm calculating the date of diabetes diagnosis is described elsewhere.62 Clinical and biochemical data to estimate trajectories following T2D diagnosis will be obtained from LABKA (Clinical Laboratory Information System Research Database).63 Information on date of various CVD and cerebrovascular disease diagnoses is based on the Danish National Patient Register,64 using International Classification of Diseases-10 codes. CVD is defined as atrial fibrillation, heart failure, hypertensive disease, IHD. Cerebrovascular disease is defined as haemorrhagic stroke, ischaemic stroke and transient cerebral ischemia. Occurrence of macrovascular atherosclerotic disease will also be available from the registers.
Data analysis plan
As described in table 1, this study will provide a wealth of data and future analyses strategies will depend on the research question and outcome in focus. The statistical methods described below serves as an example of the methods most likely to be used, while alternative approaches will be applied when appropriate.
As the established Inter99 20-year follow-up database will form the basis for testing several research questions, the analyses should be considered explorative in nature. However, a main hypothesis of the Inter99 20-year follow-up was to investigate whether LBW independent of genetics, lifestyle and glucose tolerance over 20 years is related to common T2D cardiometabolic comorbidities. For the register-based follow-up studies, Poisson regression and other time to event models (eg, Cox proportional hazards models) will be applied to estimate incidence rates and HRs with 95% CIs, respectively, of clinical outcomes like T2D and CVD. Relevant covariates such as socioeconomic factors, BMI and gene risk scores of cardiometabolic morbidity and obesity will be adjusted for in separate models. In other analysis, we will use multilevel longitudinal modelling to estimate clinical trajectories of markers of glycated haemoglobin, lipid levels, blood pressure, BMI and kidney function as a function of various lifestyle-related and perinatal (eg, birth weight) exposures.
Both binary (eg, hypertension and retinopathy), categorial (eg, sarcopenia and muscle strength) and continuous outcomes (eg, CAC score, fibrosis score, polygenetic risk scores and body composition) will be employed. We will use multiple logistic regression for binary outcomes, multinomial logistic regression for categorical outcomes and multiple linear regression for continuous outcomes. For each outcome, a series of models will be developed based on a priori knowledge about the causal framework around the association of interest. To assess the strength and direction of associations, we will report ORs and regression coefficients with corresponding 95% CIs.
We will apply causal epidemiological techniques to identify and quantify the causal relationships of various lifestyle-related, genetic and perinatal (eg, birth weight) exposures with cardiometabolic outcomes, while predictive modelling will be applied to develop algorithms that can predict future disease risk. The model and variable selection will depend on the research question. One approach is the concept of causal models and causal directed acyclic graphs. For some research questions, it is also possible to use genetic risk scores as unbiased instruments of exposures. When optimal prediction of disease is the main purpose, models will be compared by using C-statistics and other related approaches.
As the clinical relevance has been an integral part of this study from the beginning, we aim to develop a series of interactive clinical tools that apply the developed prediction models for various cardiometabolic disease outcomes such as a T2D and CVD risk engine calculators. The combination of the wealth of cardiometabolic deep phenotyping data from the ongoing 20-year follow-up, polygenetic risk scores, lifestyle factors and perinatal factors such as objectively measured birth weight in a large sample of ageing adults, provides a hitherto unparalleled potential for the development of real personalised risk prediction tools.
Patient and public involvement
None.
Ethics and dissemination
The study is conducted in accordance with the Declaration of Helsinki II and is approved by the ethical committee of the Capital Region, Denmark (Inter99 follow-up, H-20076231 ; InterVitaminK trial, H-21033114) and by the Danish Data Protection Agency (P-2020-1074). Informed consent is obtained from all participants before clinical examinations. Clinicaltrials.gov registration: NCT05166447 and NCT05259046, respectively. Examinations are considered harmless, involve minimal inconvenience and are performed by experienced healthcare professionals. Performing CT scans may result in incidental findings that need further examination and potentially treatment. As for other screening procedures, this may cause both benefit (early detection and treatment) and harm (overtreatment) to the participants. The radiation dose associated with the CT scan is relatively low and considered of minimal risk.
Findings will be disseminated in peer-reviewed journals, at national and international conferences, and via presentations to all interested stakeholders including patients and public health policy-makers. Data may be made available for international collaborations on request.
Discussion
The Inter99 cohort has contributed substantially to our understanding of the multifactorial origin, natural history, as well as potential for early detection and prevention of T2D and its associated cardiometabolic co-morbidities. While it, from a modern epidemiological perspective may appear relatively small, the Inter99 cohort is firmly established as an international competitive cardiometabolic epidemiological research resource due to its high data quality and data richness. As such, there is a strong foundation and a very high potential to perform a 20-year follow-up study of the now Inter99 participants aged 50–80 years.
So far, clinical reexaminations of the Inter99 cohort have been performed after 1, 3 and 5 years, and registry follow-up studies after 10 years. The planned 20 years follow-up study will include a combination of innovative cardiometabolic deep-phenotyping clinical examinations combined with comprehensive Danish national registry follow-up studies. While the Danish registries captures overt T2D and comorbidity diagnoses, as well as use of medications, hospital admissions, selected biochemical analyses, etc, a parallel and synergistic deep phenotyping study will allow us to determine several of the most import early disease manifestations present even prior to any official and often arbitrary cardiometabolic disease diagnosis criteria. Accordingly, the complimentary study approaches will enable us to quantify the extent to which the T2D associated comorbidities coronary arteriosclerosis, cardiac autonomic neuropathy, coronary calcification, NAFLD, retinopathy and diabetic kidney disease are present among elderly people without T2D and with a known normal glucose tolerance for two decades. The existing comprehensive genetic, birth weight and lifestyle information, collected and analysed over 20 years, provides unique opportunities to determine how individual or groups of risk factors affect the natural history of overt and/or preclinical disease manifestations during the most relevant age window with the highest occurrence rates of T2D and associated cardiometabolic diseases. The complementary data most importantly increases our signal to noise ratio, and thus statistical power, to detect the relative contribution of a variety of distinct risk factors. For instance, having near complete GWAS (Genome-wide association studies) data allows adjustment for putative genetic confounders influencing associations between birth weight and overt or preclinical disease. All pieces of information with high importance to understand the heterogeneity, and thus for driving, innovating and implementing precision medicine, of T2D and comorbidities. Compared with other prospective cardiometabolic studies including the Framingham Heart Study and Multi-Ethnic Study of Atherosclerosis, a unique feature of the Inter99 cohort, is its detailed assessment of glucose tolerance with standard 75 g oral glucose tolerance tests in all participants at the baseline examinations, as well as our broader focus on diabetes related cardiometabolic outcome variables including assessments of subclinical diabetes-related disease manifestations in arteries, liver, eye, kidney and nerves at the 20-year follow-up examinations.
The major aetiological factors underlying risk of T2D, and its comorbidities include genetics on one side, and prenatal and postnatal environmental exposures on the other (box 1). As for risk factors in pregnancy, the remarkably most accurate marker predicting cardiometabolic disease is weight at the time of birth.65 66 While there has been much focus on identifying more specific exposures underlying the association between LBW and disease risk, no single factor influencing fetal growth during pregnancy has yet been identified to explain the association. In contrast, multiple epidemiological and animal studies have documented that virtually all factors in pregnancy that negatively influence fetal growth and birth weight including maternal smoking, diet and energy intake, reduced placental blood flow, etc are associated with increased risk of cardiometabolic diseases in the offspring.67 Accordingly, rather than representing only a risk marker, LBW may represent a mediator of the totality of adverse events and lifestyle factors in pregnancy that influence later risk of cardiometabolic diseases in the offspring, justifying birth weight as the so far unparalleled cardiometabolic risk marker of prenatal disease exposures.
Based on the Inter99 baseline health examinations, we previously confirmed LBW to be associated with T2D prevalence at a mean age of only 46 years.35 As the first 20-year follow-up initiative, we studied the association between birth weight and T2D incidence rates.68 Using the Danish registries, birth records of 4590 Inter99 participants were linked with age at T2D diagnosis, as well as relevant covariates. We identified 492 new T2D cases since 1999, and subsequently documented that T2D incidence rate decreased with increasing birth weight in a surprisingly linear manner.68 Interestingly, our study clearly supported the notion that the other major aetiological factors of genetics and obesity appeared to operate as independent and most likely additive risk factors on top of that of lower birth weight.68
Further comprehensive registry analyses of the full range of T2D vascular complications and comorbidities will provide insights into previously unrecognised differential T2D and comorbidity subphenotypes and their underlying aetiologies. Here, we, for example, can determine the extent to which patients with T2D with the lowest birth weights may be characterised by a more severe clinical presentation as recently suggested.13 To improve our understanding of T2D and its subphenotypes, similar analytical strategies will be applied for the various early disease markers and manifestations determined in the deep phenotyping clinical follow-up study.
The extended InterDAG subgroup study is aiming to better understand (and adjust for) the impact of diet, whole body sodium and potassium balance, as well as physical activity, on diurnal glucose levels and fluctuations across a wide range of the glucose tolerance spectra. Blood samples available from the baseline health examinations, along with samples from the reexaminations, will be available for extended micronutrient and multiomics analyses including whole genome sequencing, metabolomics, lipidomics, transcriptomics, epigenomics, proteomics and metagenomics. Our vision includes extensive application of AI based analyses to integrate the clinical, biochemical and genetic data over time, across and beyond current clinical diagnostic cardiometabolic disease criteria.
The Inter99 20-year follow-up study furthermore provides a unique opportunity to study age-related outcomes, such as sarcopenia and physical function. It is well established that lifestyle (diet, physical activity, smoking and BMI) influences the risk of chronic disease, thus Inter99 20-year data will allow for the study of long-term impact of lifestyle in early adulthood on subsequent age-related disease manifestations. These data will, for instance, allow us to study the trends in dietary habits and physical activity patterns and their impact on muscle strength and function in middle age and old age in people with and without T2D or CVD. As such, our Inter99 follow-up study will provide important insights into the mechanisms underlying age-related processes in both healthy and diseased individuals.
An inherent limitation of the study is its observational nature that does not allow us to make strong inferences about causality. Further limitations include the fact that all individuals participated in screening for CVD risk and a personalised lifestyle intervention programme from 1999 up to 5 years thereafter, as well as the likelihood that only the healthiest cohort participants may show up for the follow-up examinations, both potentially limiting the generalisability of our findings. Finally, nearly all participants are of Danish ethnicity, as Danish literacy was a prerequisite at baseline inclusion.
In conclusion, the current combined epidemiological registry and deep phenotyping 20-year clinical follow-up study provides an example of the value of reexamining an existing and already extensively characterised T2D and cardiometabolic cohort, with the overall aim to better understand aetiologically distinct disease trajectories and subphenotypes. This will facilitate development of better and more efficacious precision medicine prediction, clinical care, as well as overall treatment approaches in T2D and associated diseases. The cohort data will via a scientific steering group be available for international collaborations.
Supplementary Material
Acknowledgments
Thank you to the Inter99 participants for their invaluable contribution to the 20-year follow-up study. We also thank our team at CCRP, Glostrup, Denmark including Thomas Meinertz Dantoft, Anne-Mette Bergmann, Pernille Hesselholt Geer, Gert Mehl Virenfeldt, Nanna Riis, Anna Stage Hansen, Emil Buch Fromberg, Tina Rasmussen, Ida Foss Engelsted, Henriette Levin, Ane Lund Hjortshøj, Karen Vrdlovec Holck, Charlotte Wibling for securing a second-to-none data collection. Evy Ottesen and Kirsten Piepgras Neergaard from Corelab, SDCC, Herlev, Denmark for providing technical assistance.
Footnotes
Contributors: KB and CB drafted the first version of the manuscript. AV and AL initiated the study. KB, CB, MA, FBK, CFBN, BL, RWC, CSH, KN, NRJ, CSH, MK, NG, JK, ML, LK, ALM, LK, KFK, RL, TH, AL and AV designed and managed the study. All authors critically reviewed the manuscript, read and approved the final version of the manuscript.
Funding: The 20-year follow-up study is initiated by Professor, Chief Physician, PhD, DMSc. Allan Vaag (co-PI) Steno Diabetes Center Copenhagen (SDCC) and Professor Allan Linneberg (co-PI) the Center for Clinical Research and Prevention (CCRP). SDCC and CCRP have funded the clinical follow-up examinations. The following institutions have further contributed to the data collection including, The Novo Nordisk Foundation Center for Basic Metabolic Research, an independent Research Center based at the University of Copenhagen, Denmark and partially funded by an unconditional donation from the Novo Nordisk Foundation (NNF18CC0034900). The Laboratory of Genomics and Biomedicine, Department of Biology, University of Copenhagen, The Institute of Sports Medicine, Bispebjerg Hospital, Copenhagen, The Danish Diabetes Academy funded by the Novo Nordisk Foundation (NNF17SA0031406).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.International Diabetes Atlas . IDF Diabetes Atlas 2021. 2021. Available: https://diabetesatlas.org/atlas/tenth-edition/ [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gedebjerg A, Almdal TP, Berencsi K, et al. Prevalence of Micro- and Macrovascular diabetes complications at time of type 2 diabetes diagnosis and associated clinical characteristics: a cross-sectional baseline study of 6958 patients in the Danish Dd2 cohort. J Diabetes Complications 2018;32:34–40. 10.1016/j.jdiacomp.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 4.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol 2022;18:525–39. 10.1038/s41574-022-00690-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vujkovic M, Keaton JM, Lynch JA, et al. Discovery of 318 new risk Loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet 2020;52:680–91. 10.1038/s41588-020-0637-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes Loci to single-variant resolution using high-density imputation and islet-specific Epigenome maps. Nat Genet 2018;50:1505–13. 10.1038/s41588-018-0241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991;303:1019–22. 10.1136/bmj.303.6809.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravelli A, van der Meulen J, Michels R, et al. Glucose tolerance in adults after Prenatal exposure to famine. The Lancet 1998;351:173–7. 10.1016/S0140-6736(97)07244-9 [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Feng X, He A, et al. Prenatal exposure to the great Chinese famine and mid-age hypertension. PLoS ONE 2017;12:e0176413. 10.1371/journal.pone.0176413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarry-Adkins JL, Ozanne SE. Nutrition in early life and age-associated diseases. Ageing Res Rev 2017;39:96–105. 10.1016/j.arr.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 11.Vaag AA, Grunnet LG, Arora GP, et al. The thrifty phenotype hypothesis Revisited. Diabetologia 2012;55:2085–8. 10.1007/s00125-012-2589-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brøns C, Thuesen ACB, Elingaard-Larsen LO, et al. Increased liver fat Associates with severe metabolic perturbations in low birth weight men. Eur J Endocrinol 2022;186:511–21. 10.1530/EJE-21-1221 [DOI] [PubMed] [Google Scholar]
- 13.Hansen AL, Thomsen RW, Brøns C, et al. Birthweight is associated with clinical characteristics in people with recently diagnosed type 2 diabetes. Diabetologia 2023;66:1680–92. 10.1007/s00125-023-05936-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007;30:1507–12. 10.2337/dc06-2537 [DOI] [PubMed] [Google Scholar]
- 15.Koo BK, Roh E, Yang YS, et al. Difference between old and young adults in contribution of Β-cell function and Sarcopenia in developing diabetes mellitus. J Diabetes Investig 2016;7:233–40. 10.1111/jdi.12392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayer AA, Syddall HE, Gilbody HJ, et al. Does Sarcopenia originate in early life? findings from the Hertfordshire cohort study. J Gerontol A Biol Sci Med Sci 2004;59:M930–4. 10.1093/gerona/59.9.m930 [DOI] [PubMed] [Google Scholar]
- 17.Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2019;393:1958–72. 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . Tackling Ncds: ‘best BUYS’ and other recommended interventions for the prevention and control of Noncommunicable diseases; 2017.
- 19.Binia A, Jaeger J, Hu Y, et al. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens 2015;33:1509–20. 10.1097/HJH.0000000000000611 [DOI] [PubMed] [Google Scholar]
- 20.Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346(apr03 3):f1378. 10.1136/bmj.f1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez V, Chang ET. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Advances in Nutrition 2014;5:712–41. 10.3945/an.114.006783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamler R. Implications of the INTERSALT study. Hypertension 1991;17(1_supplement):16–20. 10.1161/01.HYP.17.1_Suppl.I16 [DOI] [PubMed] [Google Scholar]
- 23.Stamler J, Rose G, Stamler R, et al. INTERSALT study findings. public health and medical care implications. Hypertension 1989;14:570–7. 10.1161/01.HYP.14.5.570 [DOI] [PubMed] [Google Scholar]
- 24.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34. 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 25.Kampmann FB, Thysen SM, Nielsen CFB, et al. Study protocol of the Intervitamink trial: a Danish population-based randomised double-blinded placebo-controlled trial of the effects of vitamin K (Menaquinone-7) supplementation on cardiovascular, metabolic and bone health. BMJ Open 2023;13:e071885. 10.1136/bmjopen-2023-071885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horikoshi M, The Meta-Analyses of Glucose- and Insulin-related traits Consortium (MAGIC), Early Growth Genetics (EGG) Consortium . New Loci associated with birth weight identify genetic links between Intrauterine growth and adult height and metabolism. Nat Genet 2013;45:76–82. 10.1038/ng.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warrington NM, Beaumont RN, Horikoshi M, et al. Maternal and fetal genetic effects on birth weight and their relevance to Cardio-metabolic risk factors. Nat Genet 2019;51:804–14. 10.1038/s41588-019-0403-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horikoshi M, Beaumont RN, Day FR, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature 2016;538:248–52. 10.1038/nature19806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jørgensen T, Borch-Johnsen K, Thomsen TF, et al. A randomized non-pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehabil 2003;10:377–86. 10.1097/01.hjr.0000096541.30533.82 [DOI] [PubMed] [Google Scholar]
- 30.Thomsen TF, Davidsen M, Ibsen H, et al. A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials; PRECARD(R) and the Copenhagen risk score. European Journal of Cardiovascular Prevention & Rehabilitation 2001;8:291–7. 10.1177/174182670100800508 [DOI] [PubMed] [Google Scholar]
- 31.Lau C, Vistisen D, Toft U, et al. The effects of adding group-based lifestyle counselling to individual counselling on changes in plasma glucose levels in a randomized controlled trial: the Inter99 study. Diabetes Metab 2011;37:546–52. 10.1016/j.diabet.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 32.Lau CJ, Pisinger C, Husemoen LLN, et al. Effect of general health screening and lifestyle counselling on incidence of diabetes in general population. Preventive Medicine 2016;91:172–9. 10.1016/j.ypmed.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 33.Engberg S, Vistisen D, Lau C, et al. Progression to impaired glucose regulation and diabetes in the population-based Inter99 study. Diabetes Care 2009;32:606–11. 10.2337/dc08-1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilgaard K, Færch K, Poulsen P, et al. Impact of size at birth and Prematurity on adult Anthropometry in 4744 middle-aged Danes – the Inter99 study. J Dev Orig Health Dis 2010;1:319–28. 10.1017/S2040174410000413 [DOI] [PubMed] [Google Scholar]
- 35.Pilgaard K, Færch K, Carstensen B, et al. Low birthweight and premature birth are both associated with type 2 diabetes in a random sample of middle-aged Danes. Diabetologia 2010;53:2526–30. 10.1007/s00125-010-1917-3 [DOI] [PubMed] [Google Scholar]
- 36.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, et al. New Loci associated with birth weight identify genetic links between Intrauterine growth and adult height and metabolism. Nat Genet 2013;45:76–82. 10.1038/ng.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munch IC, Larsen M, Kessel L, et al. Cumulative Glycemia and microangiopathy in subjects with impaired glucose regulation in the Inter99 study. Diabetes Res Clin Pract 2011;91:226–32. 10.1016/j.diabres.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 38.Jørgensen T, Jacobsen RK, Toft U, et al. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ 2014;348(jun09 2):g3617. 10.1136/bmj.g3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksen CU, Rotar O, Toft U, et al. What is the effectiveness of systematic population-level screening programmes for reducing the burden of cardiovascular diseases? Copenhagen: WHO Regional Office for Europe 2021. [PubMed] [Google Scholar]
- 40.Spallone V, Bellavere F, Scionti L, et al. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis 2011;21:69–78. 10.1016/j.numecd.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 41.Hansen CS, Theilade S, Lajer M, et al. Cardiovascular autonomic neuropathy and bone metabolism in type 1 diabetes. Diabet Med 2018;35:1596–604. 10.1111/dme.13777 [DOI] [PubMed] [Google Scholar]
- 42.Fuchs A, Kühl JT, Sigvardsen PE, et al. Subclinical coronary Atherosclerosis and risk for myocardial infarction in a Danish cohort A prospective observational cohort study. Ann Intern Med 2023;176:433–42. 10.7326/M22-3027 [DOI] [PubMed] [Google Scholar]
- 43.McDermott M, Newby DE. Contemporary natural history of coronary artery disease. Ann Intern Med 2023;176:574–5. 10.7326/M23-0533 [DOI] [PubMed] [Google Scholar]
- 44.Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with Sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. 10.1002/jcsm.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aadahl M, Jørgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc 2003;35:1196–202. 10.1249/01.MSS.0000074446.02192.14 [DOI] [PubMed] [Google Scholar]
- 46.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose Sarcopenia. Journal of the American Medical Directors Association 2013;14:531–2. 10.1016/j.jamda.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 47.Kück K, Isaksen JL, Graff C, et al. Spatial QRS-T angle variants for prediction of all-cause mortality. Journal of Electrocardiology 2018;51:768–75. 10.1016/j.jelectrocard.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 48.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–9. 10.1136/gut.2010.216077 [DOI] [PubMed] [Google Scholar]
- 49.Aadahl M, Beyer N, Linneberg A, et al. Grip strength and lower limb extension power in 19-72-year-old Danish men and women: the Health2006 study. BMJ Open 2011;1:e000192. 10.1136/bmjopen-2011-000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, et al. The sit-to-stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Experimental Gerontology 2018;112:38–43. 10.1016/j.exger.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 51.Byberg S, Vistisen D, Diaz L, et al. Optos wide-field imaging versus conventional camera imaging in Danish patients with type 2 diabetes. Acta Ophthalmol 2019;97:815–20. 10.1111/aos.14118 [DOI] [PubMed] [Google Scholar]
- 52.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed International clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- 53.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature 2015;521:436–44. 10.1038/nature14539 [DOI] [PubMed] [Google Scholar]
- 54.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut Microbiota. Nature 2015;528:262–6. 10.1038/nature15766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin J, Li Y, Cai Z, et al. A Metagenome-wide Association study of gut Microbiota in type 2 diabetes. Nature 2012;490:55–60. 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 56.Jie Z, Xia H, Zhong S-L, et al. The gut Microbiome in Atherosclerotic cardiovascular disease. Nat Commun 2017;8:845. 10.1038/s41467-017-00900-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39(7_suppl):30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 58.Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription Registry. Scand J Public Health 2011;39(7_suppl):38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 59.Andersen JS, Olivarius NDF, Krasnik A. The Danish national health service register. Scand J Public Health 2011;39(7 Suppl):34–7. 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 60.Jørgensen ME, Kristensen JK, Reventlov Husted G, et al. The Danish adult diabetes Registry. Clin Epidemiol 2016;8:429–34. 10.2147/CLEP.S99518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen N, Hjortdal JØ, Schielke KC, et al. The Danish Registry of diabetic retinopathy. Clin Epidemiol 2016;8:613–9. 10.2147/CLEP.S99507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carstensen B, Jørgensen ME. A Danish diabetes register. 2019. Available: http://bendixcarstensen.com/DMreg/Reg2016.pdf
- 63.Grann AF, Erichsen R, Nielsen AG, et al. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol 2011;3:133–8. 10.2147/CLEP.S17901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersen TF, Madsen M, Jørgensen J, et al. The Danish national hospital register. A valuable source of data for modern health sciences. Dan Med Bull 1999;46:263–8. [PubMed] [Google Scholar]
- 65.Risnes KR, Vatten LJ, Baker JL, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol 2011;40:647–61. 10.1093/ije/dyq267 [DOI] [PubMed] [Google Scholar]
- 66.Kajantie E, Osmond C, Barker DJP, et al. Size at birth as a Predictor of mortality in adulthood: A follow-up of 350 000 person-years. Int J Epidemiol 2005;34:655–63. 10.1093/ije/dyi048 [DOI] [PubMed] [Google Scholar]
- 67.Fernandez-Twinn DS, Hjort L, Novakovic B, et al. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019;62:1789–801. 10.1007/s00125-019-4951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wibaek R, Andersen GS, Linneberg A, et al. Low birthweight is associated with a higher incidence of type 2 diabetes over two decades independent of adult BMI and genetic predisposition. Diabetologia 2023;66:1669–79. 10.1007/s00125-023-05937-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078501supp001.pdf (127KB, pdf)