Figure 6.
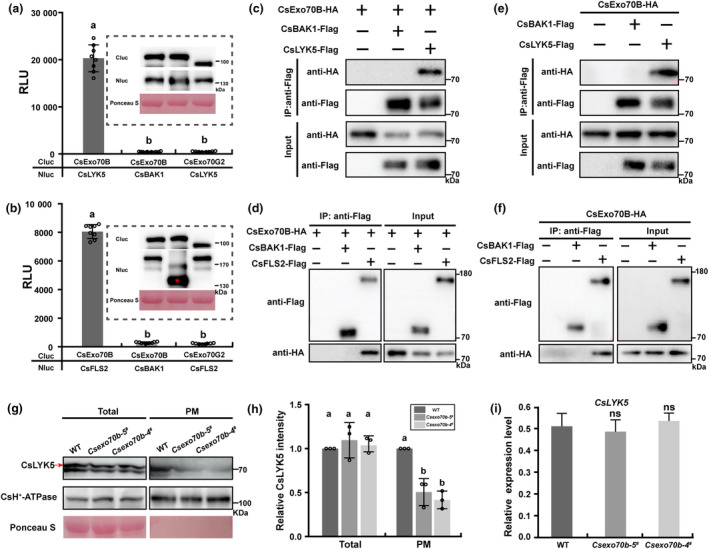
CsExo70B physically interacts with immune RKs and contributes to their accumulation at PM. (a, b) LUC assays indicated CsExo70B interacts with CsLYK5 (a) and CsFLS2 (b). The LUC assays were performed in N. benthamiana leaves by Agrobacterium‐mediated transient expression of the indicated constructs. The relative luciferase activities were measured using a luminometer. Data are shown as means ± SD; n = 8. Different lower‐case letters indicate statistically significant differences (P < 0.001, one‐way ANOVA analysis with Tukey's test). (c, d) CsExo70B associates with CsLYK5 (c) and CsFLS2 (d) in N. benthamiana. These indicated constructs were transiently expressed in N. benthamiana leaves, and co‐IP was performed by anti‐FLAG antibody. CsBAK1‐FALG was used as a negative control. (e, f) CsExo70B interacts with CsLYK5 (e) and CsFLS2 (f) in cucumber roots. The indicated constructs were expressed in hairy roots of stable CsExo70B‐HA overexpression lines, and co‐IP assays were performed with anti‐FLAG antibody. (g) Immunoblotting analysis of CsLYK5 in WT and CsExo70B knockout lines. Total proteins were extracted from true leaves and subjected to protein fractionation. CsH+‐ATPase was used as PM protein control. (h) Quantified analysis of the relative CsLYK5 levels shown in (g). The CsH+‐ATPase protein was used as control. Data represent means ± SD of three independent experiments. Different lowercase letters indicate statistically significant differences (P < 0.05, one‐way ANOVA analysis with Tukey's test). (i) The levels of CsLYK5 transcript were normal in WT and Csexo70b mutants. Data are means ± SD, n = 3 (ns, no significant difference, one‐way ANOVA).
