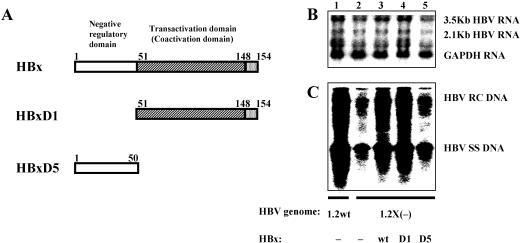
FIG. 2.
The C-terminal transactivation domain of HBx is sufficient for the stimulation effect on HBV transcription and replication. (A) Schematic representations of the HBx protein showing the locations of the amino-terminal negative regulatory domain and the carboxyl-terminal transactivation (also called the coactivation) domain (22, 23). The amino acids of the full-length HBx (154 aa residues) and the truncated HBx (HBxD1 and HBxD5, spanning aa residues 51 to 154 and 1 to 50, respectively) are shown. (B and C) HepG2 cells were transiently transfected with the wild-type HBV construct payw1.2 (1.2wt, lane 1) or the HBx-minus HBV construct payw*7 [1.2X(−), lanes 2 to 5] plus the empty vector control (−, lanes 1 and 2) or full-length HBx expression vector (wt, lane 3) or different truncated HBx expression vectors (D1, lane 4; D5, lane 5). (B) RNA (Northern) filter hybridization analysis of HBV transcripts. The GAPDH transcript was used as an internal control for RNA loading per lane. (C) DNA (Southern) filter hybridization analysis of HBV replication intermediates. HBV RC DNA, HBV relaxed circular DNA; HBV SS DNA, HBV single-stranded DNA.