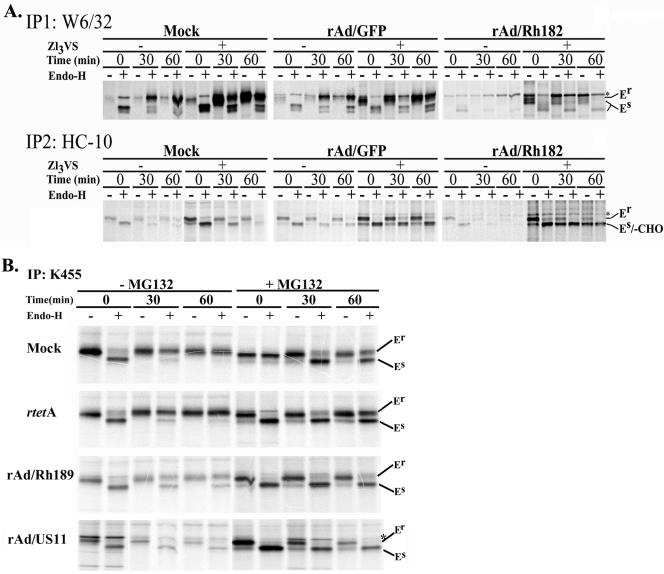
FIG. 7.
Degradation of nascent MHC I by Rh182 and Rh189. (A) Degradation of MHC I by Rh182. U373 cells were transduced with rAd/Rh182 (MOI, 100) plus rtetA (MOI, 20) or rAd/GFP (MOI, 120)for 18 h prior to metabolic labeling with [35S]Met for 15 min and chasing for the times indicated either in the absence (−) or the presence (+) of 25 μM proteasomal inhibitor ZL3VS (7). Newly assembled MHC I heterodimers were immunoprecipitated (IP) with the antibody W6/32. Remaining free heavy chains were precipitated with the monoclonal antibody HC-10 as shown. The maturation of MHC-I was assessed by EndoH digestion where indicated prior to electrophoretic separation. The gel containing rAd/Rh182 samples was exposed for twice as long as the mock and rAd/GFP samples. Note the appearance of a deglycosylated HC-reactive species in the presence of proteasome inhibitor (-CHO). The asterisks indicate a nonspecific band that comigrates with EndoH-resistant (Er) MHC I in some instances. EndoH sensitivity is labeled as in Fig. 5. (B) Degradation of MHC I molecules by Rh189. U373MG cells were transduced with rAd/US11 and rAd/Rh189 (each at an MOI of 100), together with rtetA (MOI, 20). Control cells were either mock treated or infected with rtetA (MOI, 120). Labeling conditions were as for panel A, except that the cells were pretreated for 4 h with (+) 10 μM MG132 prior to starvation and pulse-chase labeling. Both free and assembled MHC I molecules were immunoprecipitated with the polyclonal antiserum K455. The cells were treated with EndoH as indicated, except for lane 8 of the mock control, which was mistakenly not EndoH treated.