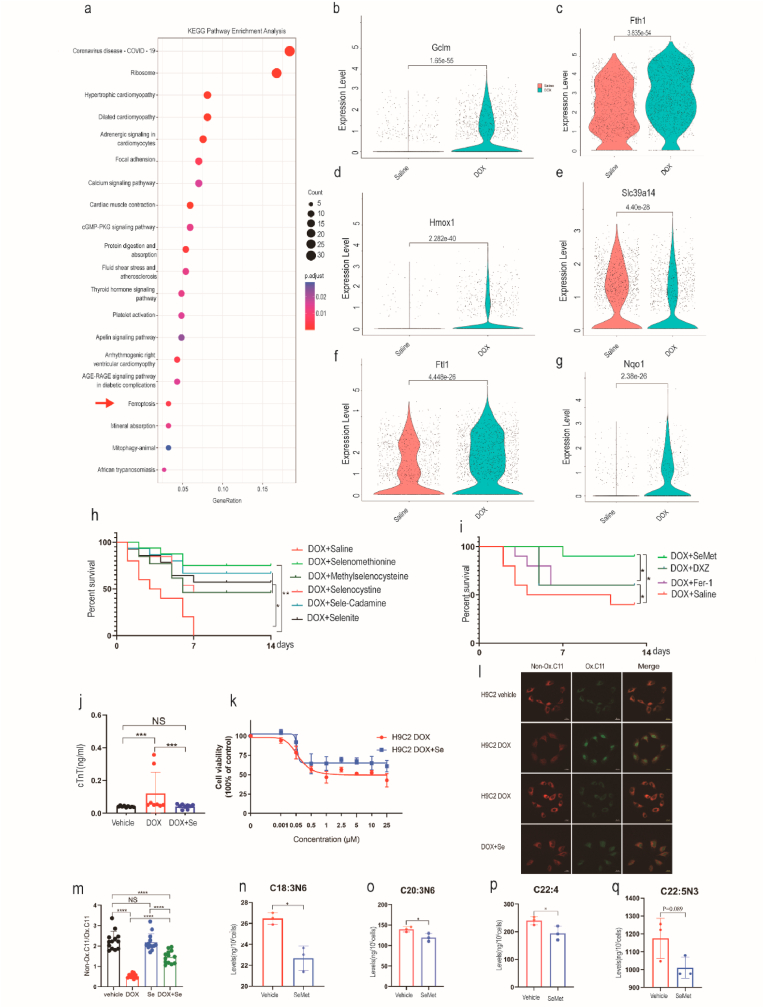
Fig. 2.
Ferroptosis is associated with DOX-induced cardiotoxicity. (a) Dot plots showing the top 20 biological terms for the DEG processes of cardiomyocytes by KEGG pathway enrichment analysis. Red arrows indicate ferroptosis-related pathways. (b–g) Violin plots showing the expression of ferroptosis-related genes in cardiomyocytes after DOX (20 mg/kg) treatment. (h) Kaplan‒Meier survival curves of mice pretreated with saline (control), SeMet, l-selenocystine, Se-methylselenocysteine, sodium selenite, and Se-rich cardamine enshiensis (0.75 mg/kg, by gavage, weekly) before DOX (20 mg/kg) administration and after the start of DOX (0.375 mg/kg, by gavage, weekly). (i) Kaplan‒Meier survival curves of mice pretreated with saline (control), Fer-1 (a ferroptosis inhibitor, 1 mg/kg), DXZ (dexrazoxane, an iron chelator, 50 mg/kg), or SeMet (a GPX4 activator), followed by DOX (15 mg/kg, i.p.) on day 0 (n = 10 mice per group). (j) Serum cTnT activity in mice on day 2 after DOX injection. DOX (20 mg/kg DOX, i.p), n = 10 mice for each group. (k) Cell viability of H9C2 cells treated with various concentrations of DOX (0 μM, 0.001 μM, 0.05 μM, 0.5 μM, 1 μM, 2.5 μM, 5 μM, 10 μM, 25 μM) or DOX + SeMet for 24 h. (l–m) C11-BODIPY 581/591 staining showing lipid peroxidation levels in H9C2 cells treated with vehicle, DOX (2 μM, 6 h), or DOX (2 μM, 6 h) + SeMet (0.1 μM). Scale bar, 20 μm. A quantitative image analysis of fluorescence median intensity was performed with ImageJ software. (n–q) Levels of selected PUFAs with the indicated treatment (n = 4). Groups were compared using one-way ANOVA or Student’s t-test. Data show the mean ± s.d. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)