Figure 2.
Generation of CD22 CART from positively and negatively selected T cells
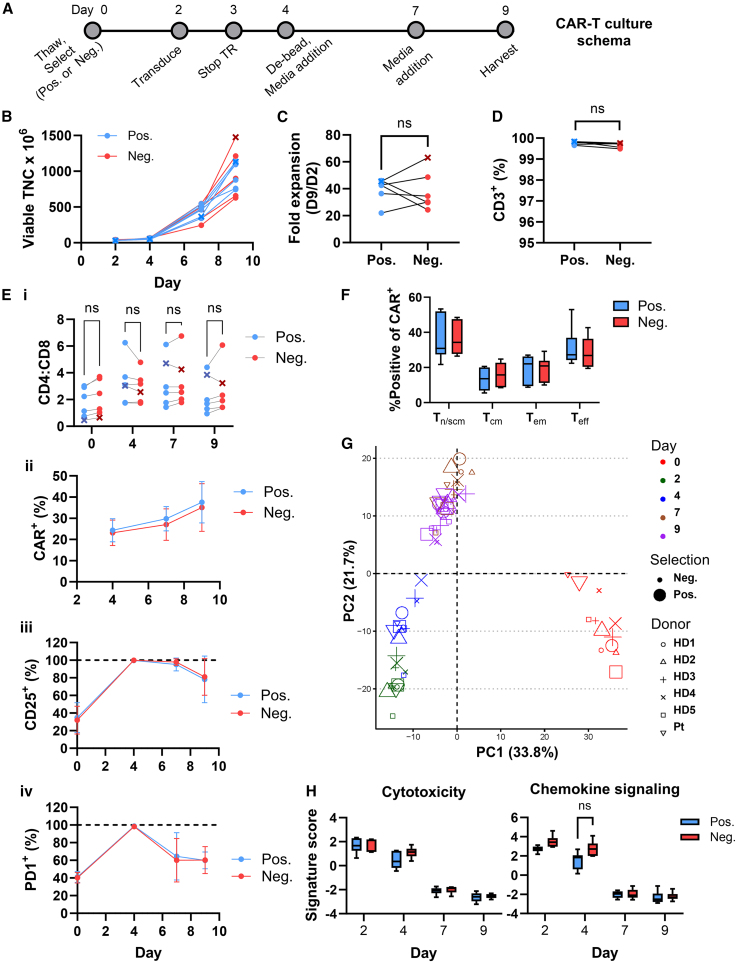
(A) Schema of the manufacturing protocol used to generate CD22 CART. TR, transduction. (B) Viable total nucleated cells (TNC) are shown between days 2 and 9 of culture. The B-ALL patient sample is indicated with an x. (C) Fold expansion was calculated as TNC on day 9 normalized to TNC on day 2. Lines are used to connect data points from the same donor. (D) CD3+ T cell purity was assessed on day 9 after gating on viable cells. (E) (i) The ratio of CD4:CD8 cells was assessed after gating on viable cells. (ii) Transduction was measured as a function of protein L staining on viable CD3+ cells, plotted as the mean ± SD of six donors. (iii) %CD25+ T cells was assessed on viable CD3+ cells, plotted as the mean ± SD of six donors. (iv) PD1 expression was assessed on viable CD3+ cells and means ± SDs of PD1+ T cells from the six donors at days 2–9 are presented. (F) Naive/stem/central memory-like (Tn/scm-like), central memory (Tcm), effector memory (Tem), and terminal effector (Teff) T cells were monitored by flow cytometry as CCR7+CD45RA+, CCR7+CD45RA−, CCR7−CD45RA−, and CCR7−CD45RA+, respectively and gated from the CD3+CAR+ population. Day 9 means ± SEM (n = 6) are shown. (G) PCA and (H) pathway analysis of gene expression data assessed at days 2, 4, 7, and 9 of culture. Means ± SEM for the six donors are shown. Statistical significance was calculated using a multiple paired t test; ns > 0.05.