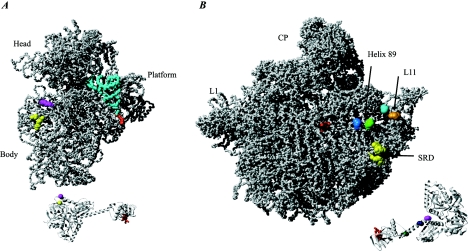
FIG. 10.
IF2 Interactions with fMet-tRNAfMet and the ribosome. (A) The 30S ribosomal subunit from T. thermophilus (PDB entry 1J5E) in complex with a P-site fMet-tRNAfMet (derived and docked based on PDB entry 1GIX). The fMet-ACC region of the tRNA that interacts with the C-terminal region of IF2 is shown in red. The corresponding region is red on the aIF5B structure shown at the bottom part (PDB entry 1G7T). Two residues in domain V of IF2 (V451 and S520 of B. stearothermophilus IF2) are shown in yellow and magenta, respectively. A nuclease at position V451 cleaves positions 38 to 40 and 498, and a nuclease at position S520 cleaves positions 538 to 540 of the E. coli 16S rRNA in a 70S ribosomal complex. The corresponding positions are indicated in yellow and magenta on the ribosomal subunit. Note that these cleavages are absent in a 30S-IF2 initiation complex. (B) The 50S ribosomal subunit from H. marismortui (PDB entry 1JJ2) is shown along with aIF5B. Red indicates the position of the fMet ACC in the decoding center of the ribosomal subunit and the corresponding region on a IF5B that interacts with the fMET ACC. Pale blue and light green on the subunit indicate two positions in helix 89 of the 23S rRNA (U2474 and A2482 in E. coli numbering) that are cleaved by nucleases attached at the residue located at the interface between domains VI-1 and VI-2 of IF2 (shown in dark green on the aIF5b structure) (E644 of the B. stearothermophilus IF2). A nuclease attached to a residue in domain VI-1 of IF2 (dark blue) (E632 of the B. stearothermophilus IF2) cleaves both the C1076 (cyan) and G1068 (cyan) positions in the L11 region. The nuclease (shown in purple) on aIF5B (position Y625 of the VI-1 domain) cleaves weakly at C1076 (cyan) and U2474 (light blue). Data were derived from reference 121. Another study showed that IF2 protects residues in the sarcin-ricin domain (SRD) (G2655, A2665, and G2661) against chemical modification (100). These residues are indicated in yellow on the subunit. No attempt was made to dock IF2 on the ribosomal subunit, since it is likely that conformational changes occur in the subunit as a result of IF2 binding.