FIG. 12.
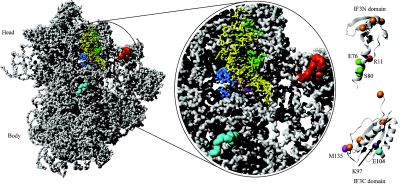
Interaction of IF3 with the 30S ribosomal subunit. The interactions between IF3 and the 30S ribosomal subunit identified by hydroxyl radical footprinting and directed probing are shown (data from reference 42). (A) 30S ribosomal subunit from T. thermophilus with P-site-bound tRNA (derived from PDB entries 1J5E and 1GIX). The tRNA is shown in yellow. (B) Close-up of the ribosomal subunit. Sites in the 16S rRNA and on the tRNA that are cleaved by nucleases attached to IF3 are indicated. The IF3N and IF3C domains (PDB entries 1TIF and 2IFE) are shown in a ribbon representation, with the modified cysteines indicated by spheres. Orange spheres on the IF3 domains indicate residues where an attached nuclease does not cleave the 16S rRNA or tRNA. The magenta spheres in the IF3C domain indicate residues K97 and MI35 from where nucleases cleave in the 790 loop of the 16S rRNA (magenta) seen below the acceptor arm of the tRNA. Nucleases at these positions also cleave the side of the tRNA marked in blue (residues 3 to 5 and 12 to 24). The cyan sphere in the IF3C domain indicates E104 from which nucleases cleave in the 790 loop (magenta) and at residues 1482 to 1487, indicated in cyan on the 30S subunit. Green spheres in the linker region on the IF3N domain indicate residues (E76 and S80) on which nucleases cleave on the other side of the tRNA, marked in green (residues 26 to 29 and 35 to 37), the 790 loop region (magenta), and the 690 loop region (red) of the 16S rRNA. A nuclease attached to position R11 in the IF3N domain (red) cleaves at positions in the 690 loop (red). No attempt was made to dock IF3 on the ribosomal subunit, since conformational changes most probably take place in the subunit as a result of IF3 binding.