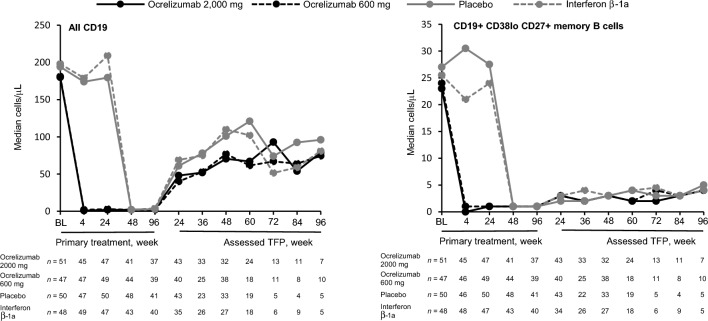
Fig. 2.
Median overall CD19 cell and CD19+ CD38lo CD27+ memory B-cell counts during the primary treatment and assessed treatment-free periods, by initial randomization group. From week 24 to week 96 of the PTP, all patients were on ocrelizumab. Almost all post-baseline medians for predose CD19 in the OLE (OCR 600 mg) were < 5 cells/µL and are not shown. Assessed TFP assessed treatment-free period, BL baseline, OCR ocrelizumab, OLE open-label extension, PTP primary treatment period