Fig. 4.
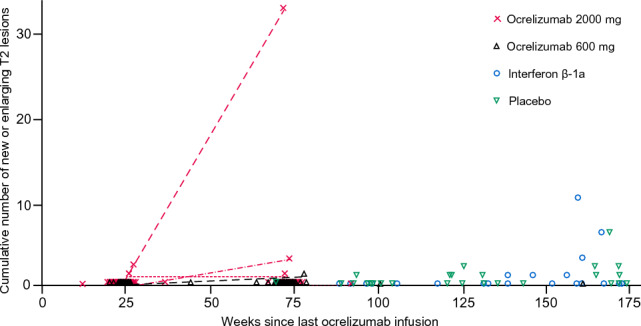
Cumulative number of new or enlarging T2 lesions by treatment after last ocrelizumab infusion up to and including the OLE baseline. Patients included had a 24-week MRI assessment and received at least three cycles of ocrelizumab during PTP. Participants randomized at baseline to receive ocrelizumab 2000 mg or 600 mg had scans at week 96 (i.e. 24 weeks after the last ocrelizumab treatment) and at week 144; other patients received an MRI at baseline OLE, which was compared with the 24-week MRI. Dots show all assessments performed; lines connect patients with more than one MRI assessment. One patient (randomized to the ocrelizumab 2000 mg group) had 32 new or enlarging T2 lesions and 11 T1 Gd-enhancing lesions 71 weeks after the last ocrelizumab infusion. This patient had three relapses during the assessed TFP (a clinical relapse at 67 weeks, and two protocol-defined relapses at 94 and 126 weeks, respectively, after the last ocrelizumab infusion). One patient (randomized to the ocrelizumab 600 mg group) had one new or enlarging T2 lesion and one T1 Gd-enhancing lesion at 78 weeks since last ocrelizumab infusion. One patient (randomized to the placebo group) had two new or enlarging T2 lesions and one T1 Gd-enhancing lesion at week 171 since last ocrelizumab infusion. Assessed TFP assessed treatment-free period, Gd gadolinium, OLE open-label extension
