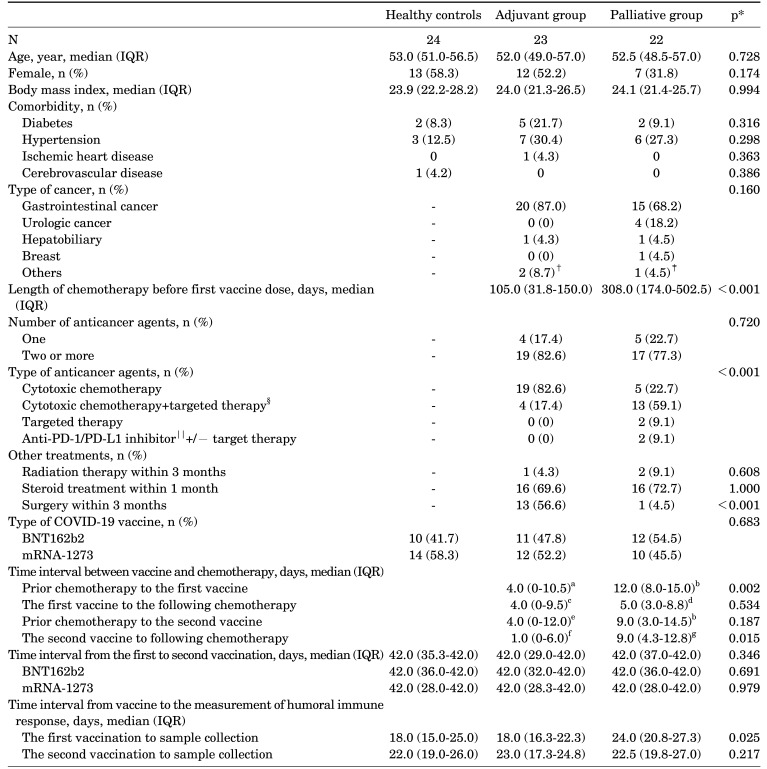
TABLE 1. Demographic and clinical characteristics of healthy controls and patients with solid cancers receiving adjuvant or palliative chemotherapy.
*Control vs. adjuvant vs. palliative chemotherapy group or adjuvant vs. palliative chemotherapy group. Comparisons of categorical variables were performed using Fisher exact tests or chi-square tests. Mann-Whitney or Kruskal-Wallis tests were used for comparing continuous variables. A two-sided p-value<0.05 was considered statistically significant; †Oropharyngeal cancer and non-small cell lung cancer; ‡nasopharyngeal cancer; §bevacizumab, cetuximab, aflibercept, pazopanib, everolimus; ∥nivolumab, atezolizumab. an=18, one patient received the first vaccine before chemotherapy started. Data of 4 patients for chemotherapeutic agent administration day were not available; bn=21, data of one patient for chemotherapeutic agent administration day were not available; cn=18, one patient no longer received chemotherapy after the first vaccine. Data of 4 patients for chemotherapeutic agent administration day were not available; dn=20, one patient no longer received chemotherapy after the first vaccine. Data of one patient for chemotherapeutic agent administration day were not available; en=19, data of 4 patients for chemotherapeutic agent administration day were not available; fn=16, three patients did not received chemotherapy after the second vaccine. Data of 4 patients for chemotherapeutic agent administration day were not available; gn=20, one patient did not received chemotherapy after the second vaccine. Data of one patient for chemotherapeutic agent administration day were not available. COVID-19: Coronavirus disease 2019, IQR: interquartile range, N, n: number, PD-1: programmed cell death protein 1, PD-L1: programmed death-ligand 1.